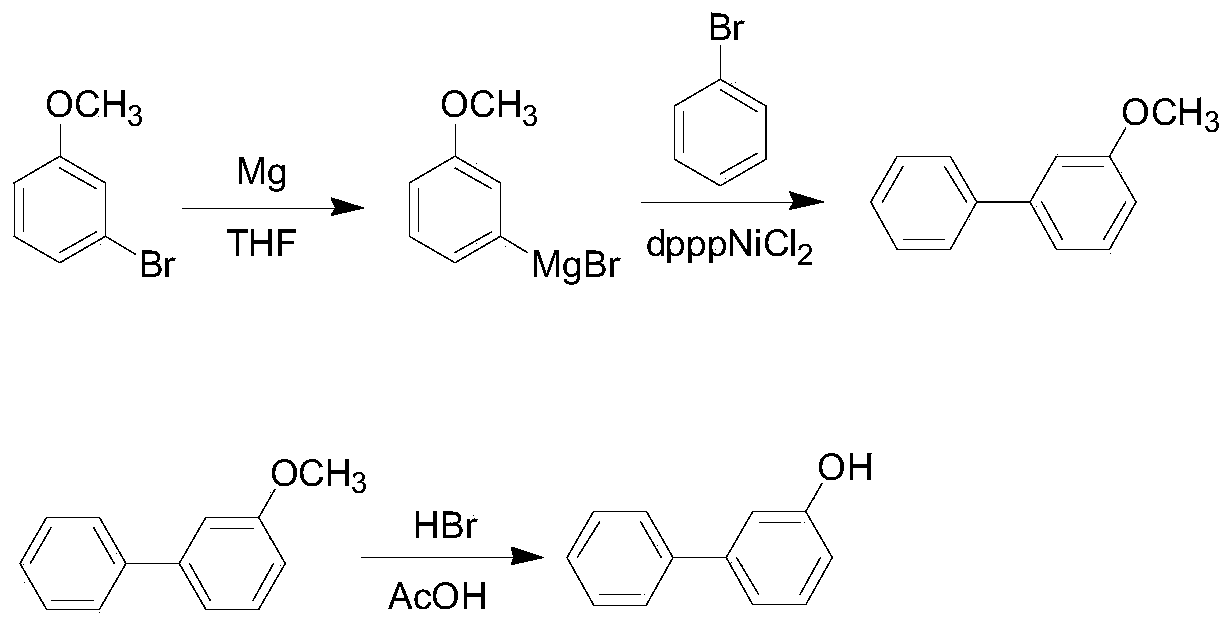
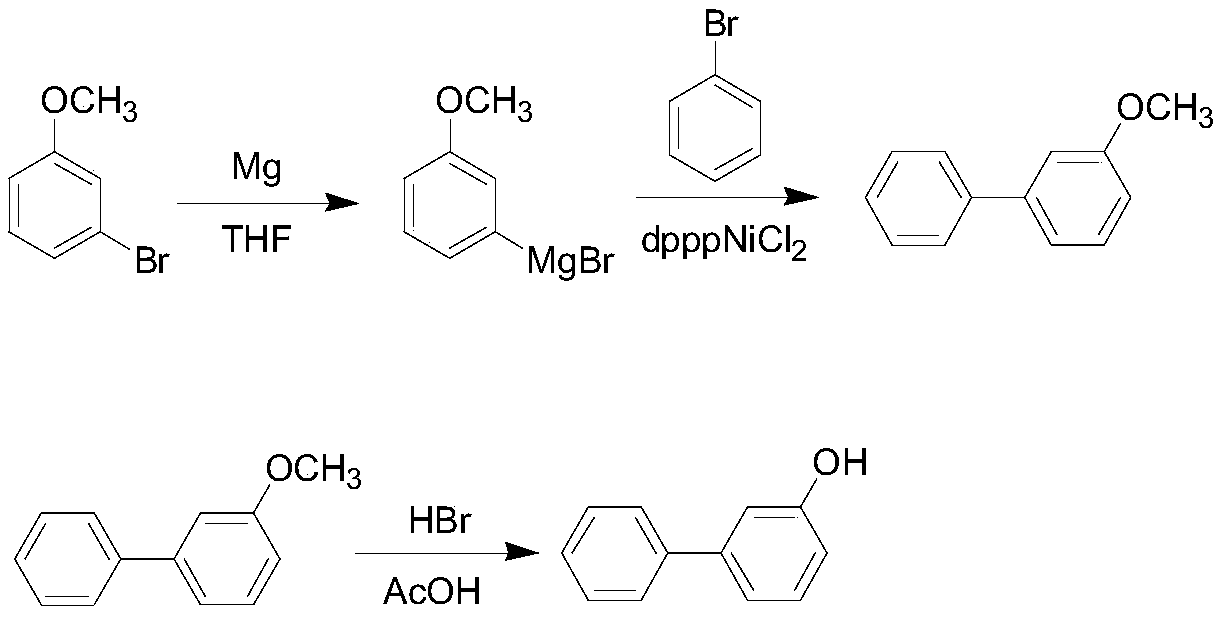
Method for synthesizing 3-hydroxy biphenyl
A technology of hydroxybiphenyl and synthesis method, which is applied in chemical instruments and methods, magnesium organic compounds, preparation of organic compounds, etc., can solve the problems of cumbersome processing, high cost, expensive, etc., and meet the requirements of simple reaction equipment and easy control of reaction , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment provides a process for synthesizing 3-hydroxybiphenyl, and the specific synthesis steps are as follows:
[0031] (1) prepare 10L glass reactor, N 2 protection, adding magnesium scraps (171g, 7.12mol) and 800ml solvent THF in sequence, then heating to 62-64°C, adding dropwise a THF (2L) solution of 3-bromoanisole (1.27kg, 6.78mol) to keep a slight Add dropwise under boiling conditions. After the dropwise addition, mature at 62-65°C for 1 hour to react 3-bromoanisole with magnesium chips to obtain Grignard reagent;
[0032] (2) Cool the Grignard reagent prepared in step (1) to 5-10°C in an ice-water bath, and then add the catalyst dpppNiCl first 2 (3.5g, 6.45mmol), then add bromobenzene (845g, 5.38mol) dropwise, the rate of addition is controlled within 1.0-1.5h, after the dropwise addition, first react at an internal temperature of 40°C for 1h, then reflux at 68°C for 3h; After the reaction, cool the reaction solution to 5-10°C, add 600ml concentrated h...
Embodiment 2
[0040] This embodiment provides a process for synthesizing 3-hydroxybiphenyl, and the specific synthesis steps are as follows:
[0041] (1) prepare 10L glass reactor, N 2 protection, adding magnesium chips (179g, 7.46mol) and 800ml solvent THF in sequence, then heating to 62-64°C, adding dropwise a THF (2L) solution of 3-bromoanisole (1.27kg, 6.78mol) to keep a slight Add dropwise under boiling conditions. After the dropwise addition, mature at 62-65°C for 1 hour to react 3-bromoanisole with magnesium chips to obtain Grignard reagent;
[0042] (2) Cool the Grignard reagent prepared in step (1) to 5-10°C in an ice-water bath, and then add the catalyst dpppNiCl first 2 (3.5g, 6.45mmol), then added dropwise bromobenzene (1064g, 6.78mol), the rate of addition was controlled within 1.5-2.0h, after the dropwise addition, the internal temperature was first reacted at 40°C for 1h, and then refluxed at 68°C for 3h; After the end, cool the reaction solution to 5-10°C, add 600ml concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com