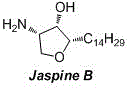
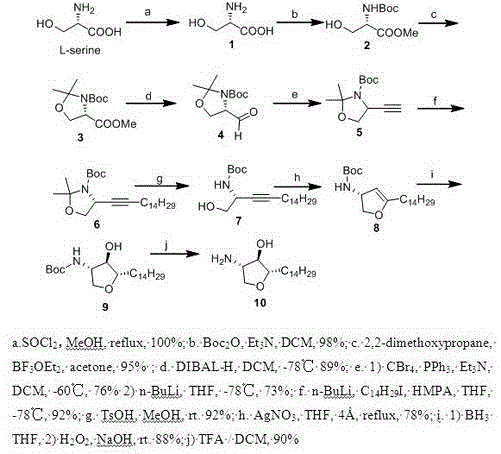
Method for synthesizing natural product Jaspine B isomer
A technology for natural products and isomers, applied in the production of bulk chemicals, organic chemistry, etc., which can solve the problems of slow speed, easy racemization of chiral centers, and long synthetic routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Synthesis of Compound of Formula 1
[0029] L - Serine (4.2g, 40mmol) was dissolved in 200mL of methanol, cooled to 0°C, and a solution of thionyl chloride (3.2mL, 44mmol) was added. The solution was heated slowly to reflux, then refluxed overnight. After the reaction was complete, the solution was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a white crystalline solid, i.e. L - Serine methyl ester hydrochloride 1 (6.1 g, 100% yield).
[0030] Synthesis of Formula 2 Compounds
[0031] Will L - Serine methyl ester hydrochloride 1 (6.0g, 38.7mmol) was dissolved in 200mL of dichloromethane, cooled to 0°C, 2mL of triethylamine was added dropwise, and stirred for 5 minutes. Then Boc anhydride (8.7 g, 38.7 mmol) was added dropwise. After stirring for 10 minutes, the ice-water bath was removed and the suspension was stirred overnight at room temperature. After the solvent was removed under reduced pressure, saturated a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com