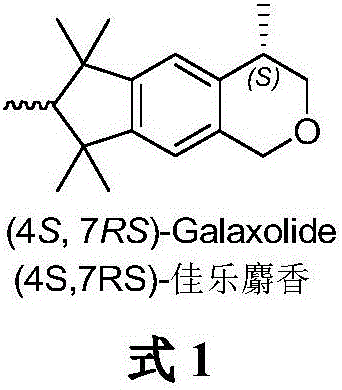
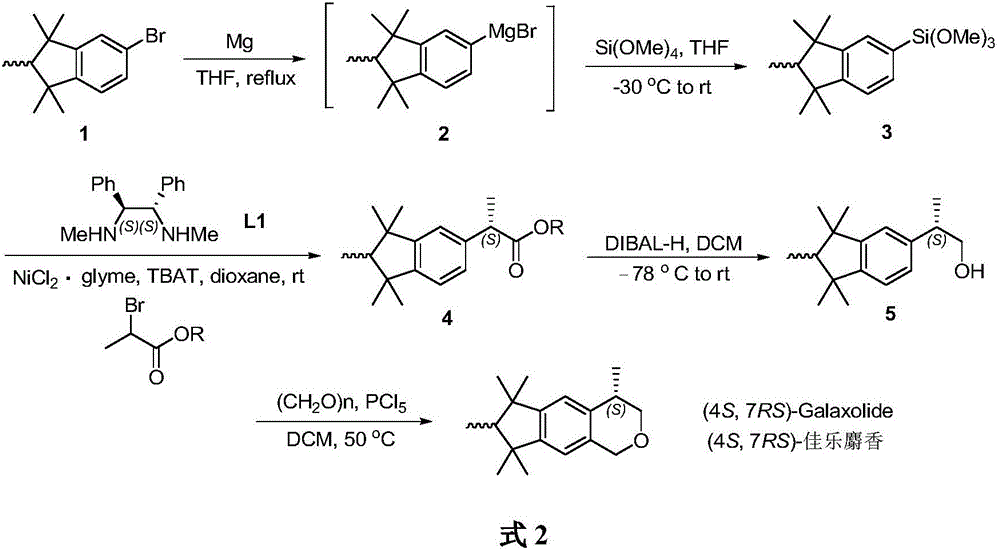
(4S, 7RS)-galaxolide synthesis method
A kind of technology of Jiale musk and synthetic method, applied in the direction of organic chemistry method, organic chemistry, etc., can solve the problem of complicated synthetic route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of Pentamethylindane Silane
[0019] Under argon protection, magnesium powder (0.23 g, 9 mmol) was added to a 20 mL Schlenk reaction flask, and dried in vacuum for 2 h. Bromopentamethylindane (0.54g, 2mmol) was added in tetrahydrofuran 2mL, the mixture was heated slowly at 50°C to initiate the reaction, and then the remaining bromopentamethylindane (1.07g, 4mmol) in tetrahydrofuran was slowly added Solution 4mL. After adding the bromide, the reaction mixture was carefully heated to reflux, and continued heating and stirring for 5h to obtain the pentamethylindane Grignard reagent.
[0020] Under argon protection, tetramethoxysilane (2.74 g, 18 mmol) and tetrahydrofuran (10 mL) were added into a 50 mL Schlenk reaction flask, and stirred evenly. The temperature of the mixture was lowered to -30°C, and then 6 mL of a tetrahydrofuran solution of pentamethylindane Grignard reagent was added dropwise. The reaction was stirred at -30°C for 1 h, then slowly warmed t...
Embodiment 2
[0022] Synthesis of (S)-hexamethylindanate
[0023] Under the protection of argon, add NiCl to the 50mL Schlenk reaction flask 2 .glyme (22.0mg, 0.1mmol), [F 2 SiPh 3 ] - [NBu 4 ] + (TBAT, 1080mg, 2.0mmol) and (1S,2S)-N,N-dimethyl-1,2-diphenylethylenediamine (28.8mg, 0.12mmol), then add dioxane (20mL) . The mixture was stirred for 10 min, and pentamethylindansilane (401 mg, 1.3 mmol) and 2-bromopropionic acid-2,6-di-tert-butyl-4-methylphenyl ester (355.3 mg, 1 mmol) were added. The reaction mixture was stirred for 18 h at room temperature. Add a mixed solution of 1M hydrochloric acid and acetone (1:1, 10mL), and stir for 2h. The mixture was poured into water (50 mL), and the organic phase was separated. The aqueous phase was extracted with diethyl ether (3 x 50 mL). The combined organic phases were washed with saturated NaCl solution (50 mL). anhydrous MgSO 4Dry and concentrate under reduced pressure to obtain the crude product. Finally, it was purified by silica ...
Embodiment 3
[0025] Synthesis of (S)-hexamethylindanol
[0026] Under argon protection, add anhydrous CH to a 20 mL Schlenk reaction flask 2 Cl 2 (10 mL), add (S)-hexamethylindanate (1.85 g, 5 mmol), and stir well. The temperature of the mixture was lowered to -78°C, and diisobutylaluminum hydride (DIBAL-H) (7.5 mL, 1.5M solution in toluene, 11 mmol) was added slowly. After the dropwise addition, the stirring reaction was continued at -78°C for 30 minutes, and then the temperature of the reaction mixture was raised to 0°C, and the reaction was stirred for another 30 minutes. After the reaction, the temperature of the reaction mixture was lowered to -78°C, and the reaction was quenched with methanol (1 mL). An aqueous solution of potassium sodium tartrate (22 mL, 0.5 M, 11 mmol) was added, the temperature of the resulting mixture was slowly raised to room temperature, and stirring was continued for 12 h. Separate the organic phase and the aqueous phase with CH 2 Cl 2 (3 x 20 mL) ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com