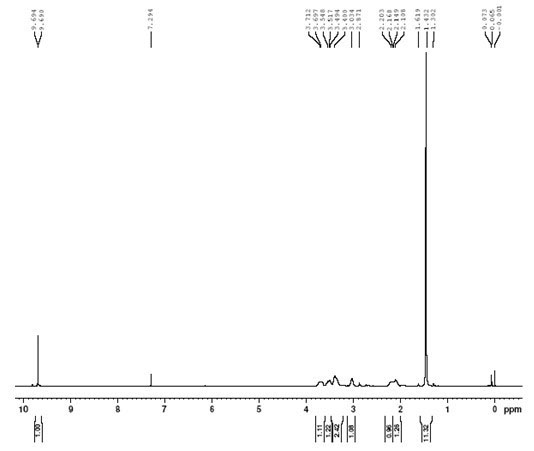
Method for synthesizing N-Boc-3-pyrrolidine formaldehyde
A technology of n-boc-3-, pyrrolidine carboxaldehyde, applied in the direction of organic chemistry and the like, can solve the problems of many reaction steps, difficult to scale up production, difficult to operate, etc., and achieve high yield, easy operation, and few steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Add 1 mol of glycine and 1 mol of acrylonitrile into 1000 ml of toluene as a solvent, under the action of 2 mol of paraformaldehyde, reflux for 3 hours, filter with suction after cooling down, concentrate the mother liquor, distill under reduced pressure with a water pump, and collect fractions at 102-105 degrees to obtain 3- Cyanopyrrolidine, yield 85%, purity 98%;
[0019] (2) Add 0.5 mol of 3-cyanopyrrolidine obtained in step (1) into 500 ml of dichloromethane, under the action of 0.55 mol of triethylamine, add 0.5 mol of Boc anhydride dropwise at room temperature, and react at room temperature for 12 hours; then wash the reaction with hydrochloric acid solution liquid, separated, dried, and concentrated to obtain N-Boc-3-cyanopyrrolidine with a yield of 90% and a purity of 97%;
[0020] (3) Dissolve 0.4mol of N-Boc-3-cyanopyrrolidine obtained in step (2) in 400ml of dichloromethane, cool down to minus twenty degrees, add 0.5mol of diisobutylaluminum hydride drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com