Novel synthesis method for key intermediate of anti-hepatitis B drug Entecavir
A technology of entecavir and synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of harsh operating conditions, high price of entecavir, long synthesis route and the like, and achieves the effects of less side reactions, unique and novel design, and fast speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
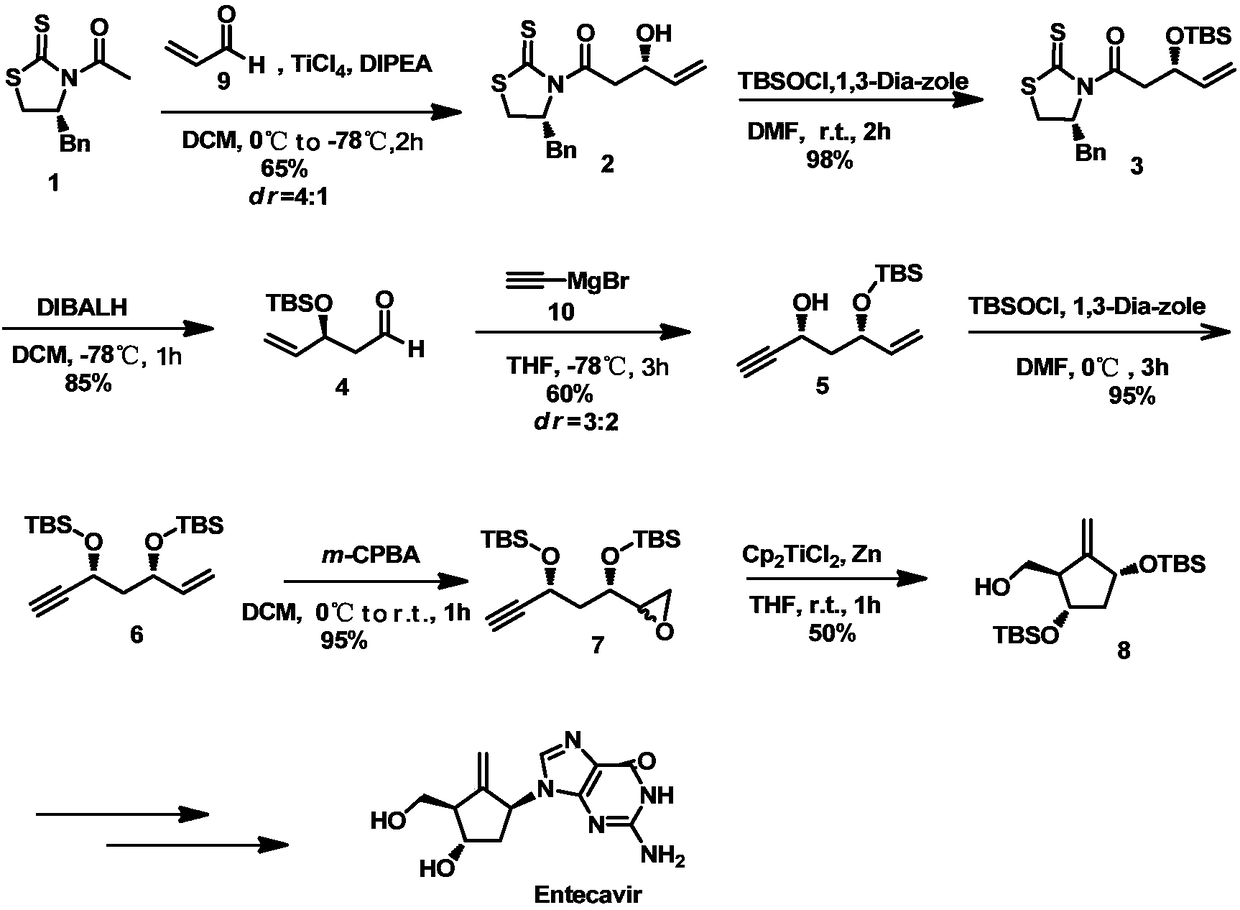
[0034] 1) Synthesis of formula 2 compound:
[0035] At 0°C and under the protection of nitrogen, titanium tetrachloride (1.2 mL) was slowly added dropwise to a solution of the compound of formula 1 (2.5 g, 9.97 mmol) in dichloromethane (60 mL), the solution turned yellow and stirred for 5 minutes, At this temperature, diisopropylethylamine (1.9mL) was continued to be added dropwise, and after the dropwise addition, the flask was transferred to -78°C for reaction, and the solution quickly turned dark red; the compound of formula 9 (0.51g, 9.06 mmol) of dichloromethane (7mL) solution was slowly injected into the above solution, and after reacting for 2 hours, a saturated ammonium chloride solution (10mL) was added to the mixed system, extracted with dichloromethane (3×80mL), and the resulting mixture was combined The organic phase was washed with saturated brine and dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the organic solvent, and the ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com