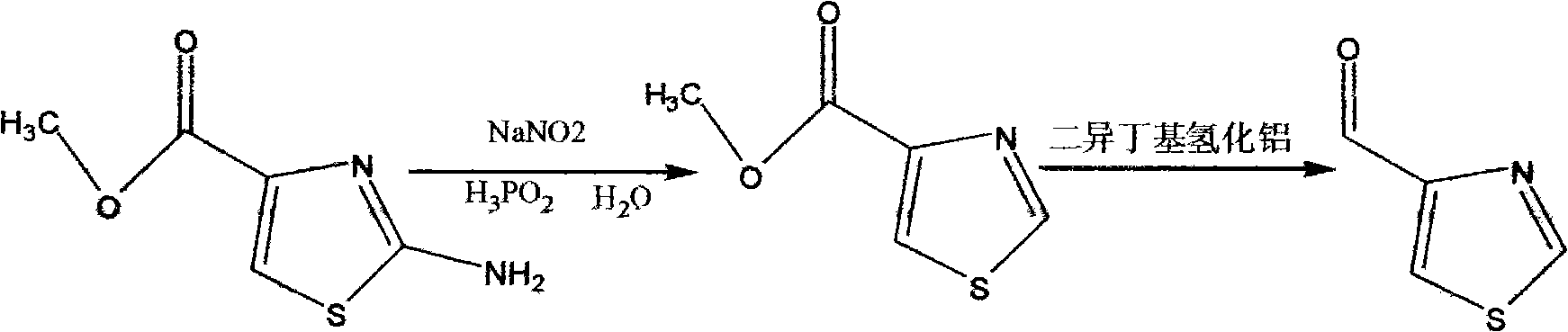
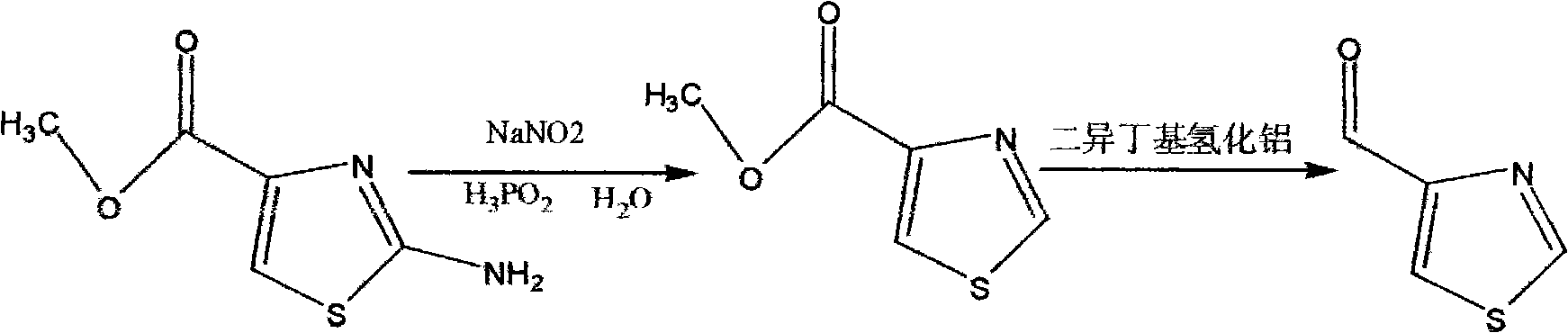
Synthesis method of thiazole-4-formaldehyde
A synthesis method and thiazole technology are applied in the field of synthesis of chemical product thiazole-4-carbaldehyde, which can solve the problems of high reaction temperature, high cost of raw materials, and difficult preparation and production methods, and achieve simple preparation operations, simple preparation methods, Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Add 396 g (3.0 MOL) of hypophosphorous acid (50%) and 79 g (0.5 MOL) of methyl 2-amino-thiazole-4-carboxylate to a 2 L three-necked flask in sequence, and stir for 5 minutes, at which point all the solids are dissolved. Cool the system down to -10°C ± 5°C and start adding 52g of sodium nitrite aqueous solution dropwise, controlling the temperature between -15°C and 15°C. After the dropwise addition, the temperature was naturally raised to 0°C, and after stirring for 2 hours, the reaction was stopped. The reaction solution was poured into a 5L beaker, and 125g of ice was added, followed by 275mL of sodium hydroxide aqueous solution to adjust the pH to 7.0. After stirring for 30 minutes, ethyl acetate was added. 2L of ester, stirred for 20min, added hot brine at 70°C to 75°C, stirred for 30min, allowed to stand to separate the organic phase, extracted the aqueous phase with 2L of ethyl acetate, combined all organic phases and dried with 180g of anhydrous magnesium sulfate ...
Embodiment 2
[0014] Add 3168g (50%) hypophosphorous acid (50%) (24.0MOL) and 632g (4.0MOL) of methyl 2-amino-thiazole-4-carboxylate to a 10L three-necked flask in sequence. After stirring for 5min, all the solids are dissolved. Cool the system down to -10°C ± 5°C and start adding 416g of sodium nitrite aqueous solution dropwise, controlling the temperature between -15°C and 15°C. After the dropwise addition, the temperature was naturally raised to 0°C, and after stirring for 2 hours, the reaction was stopped, and the reaction solution was poured into a 30L plastic bucket. First, 1000g of ice was added, and then 2200mL of sodium hydroxide aqueous solution was added to adjust the pH to 7.0. After stirring for 30 minutes, acetic acid was added. 13L of ethyl ester, stir for 20min, add hot brine at 70°C to 75°C, stir for 30min, let stand to separate the organic phase, extract the water phase with 13L of ethyl acetate, combine all organic phases and dry with 1500g of anhydrous magnesium sulfate f...
Embodiment 3
[0017] Add 2000 mL of dichloromethane and 286 g (2.0 MOL) of methyl thiazole-4-carboxylate to a 5 L three-necked flask in sequence, and pass through nitrogen protection. After stirring for 15 minutes, the system is completely dissolved. After the system was cooled to -75°C, 1670 g (2.94 MOL) of diisobutylaluminum hydride solution (25%) was added dropwise, the temperature of the system was controlled to react for 2 hours between -72°C and -78°C, and the reaction was stopped. Cool the system down to -80°C, stop stirring, pour the reaction solution into a 30L plastic bucket, slowly add 620mL of isopropanol, 930mL of water, and 1000mL of dichloromethane under stirring, and then add 1000mL of dichloromethane after stirring for 1 hour. Filter, rinse the filter cake twice with dichloromethane (1500mL, 1000mL), concentrate the organic phase to obtain 220g of a light yellow solid, add 220mL of dichloromethane to the light yellow solid, heat to 40°C to completely dissolve the system, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com