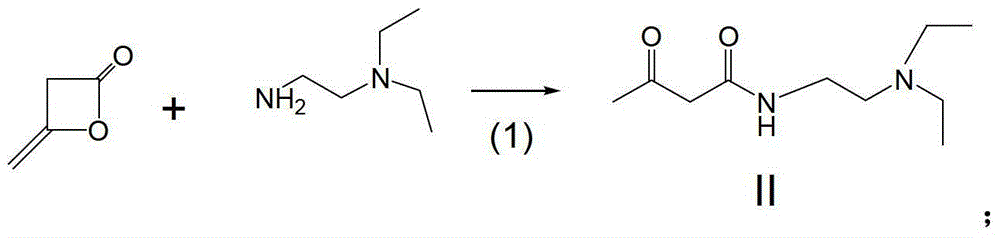A kind of preparation method of sunitinib intermediate
A sunitinib and intermediate technology, applied in the field of medicine, can solve the problems of high synthesis cost, difficult promotion, strong corrosion, etc., and achieve the effects of simplifying the reaction process, reducing the pollution of three wastes, and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
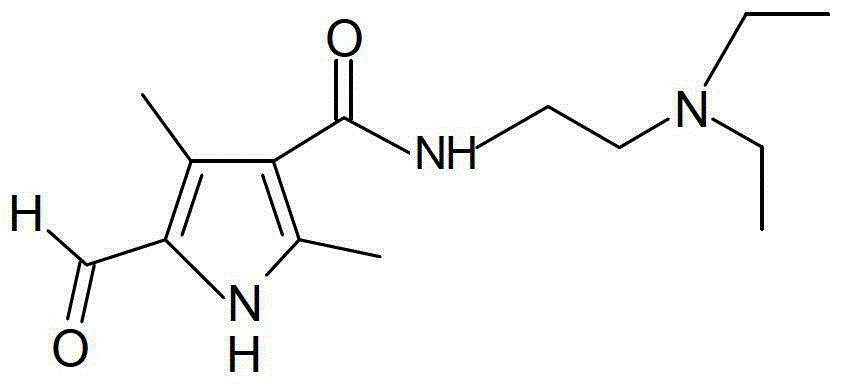
[0036] This example provides a preparation method of sunitinib intermediate (compound I), which specifically includes the following steps:
[0037] (1) Weigh 84g (1mol) of diketene, add 200ml of purified water, stir at room temperature, then add 139g (1.2mol) of N,N-diethylethylenediamine dropwise for about 1 hour. Stirring was continued for 10 hours at room temperature. Water, unreacted diketene and N,N-diethylethylenediamine were distilled off under reduced pressure, and the residue was viscous.
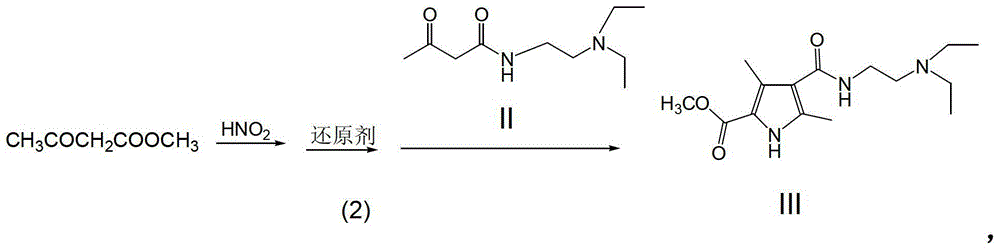
[0038] (2) Weigh 116g (1mol) of methyl acetoacetate, add 200ml of glacial acetic acid, and stir. Add NaNO dropwise 2 solution (NaNO 2 151g (2.2mol) + 200ml of water], drop it in about 1 hour. Stirring was continued for 3 hours at room temperature, and then 112 g (2 mol) of iron powder was added in batches. Reflux for 3 hours, filter out the insoluble matter while it is hot, add the filtrate to the sticky silk obtained in step (1), stir evenly, heat and reflux for 4 hours, and ...
Embodiment 2
[0043] This example provides a preparation method of sunitinib intermediate (compound I), which specifically includes the following steps:
[0044] (1) Weigh 84g (1mol) of diketene, add 200ml of purified water, stir at room temperature, then add 139g (1.2mol) of N,N-diethylethylenediamine dropwise for about 30 minutes. Stirring was continued for 8 hours at room temperature. Water and unreacted diketene and N,N-diethylethylenediamine were distilled off under reduced pressure. The residue was viscous.
[0045] (2) Weigh 116g (1mol) of methyl acetoacetate, add 250ml of glacial acetic acid, and stir. Add NaNO2 solution [NaNO2137g (2mol) + water 200ml] dropwise, and drop it in about 1 hour. Stirring was continued at room temperature for 3 hours, and then 112 g (2 mol) of iron powder (Fe) was added in batches. Reflux for 3 hours, filter out the insoluble matter while it is hot, add the filtrate to the sticky silk in step (1), stir evenly, heat and reflux for 4 hours, and finally...
Embodiment 3
[0048] This example provides a preparation method of sunitinib intermediate (compound I), which specifically includes the following steps:
[0049] (1) Weigh 84g (1mol) of diketene, add 200ml of purified water, stir at room temperature, then add 139g (1.2mol) of N,N-diethylethylenediamine dropwise, and the dropwise addition time is about 45 minutes. Stirring was continued for 8 hours at room temperature. Water and unreacted diketene and N,N-diethylethylenediamine were distilled off under reduced pressure. The residue was viscous.
[0050] (2) Weigh 116g (1mol) of methyl acetoacetate, add 280ml of glacial acetic acid, and stir. Add NaNO2 solution [NaNO2151g (2.2mol) + water 200ml] dropwise, and drop it in about 45 minutes. Stirring was continued for 4 hours at room temperature, and then 130 g (2 mol) of zinc powder (Zn) was added in batches. Reflux for 3 hours, filter out the insoluble matter while it is hot, add the filtrate to the sticky silk in step (1), stir evenly, hea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com