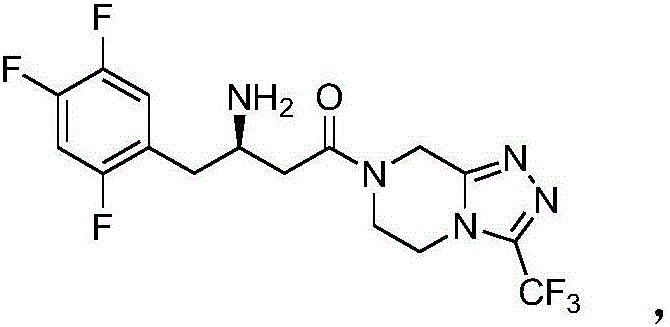
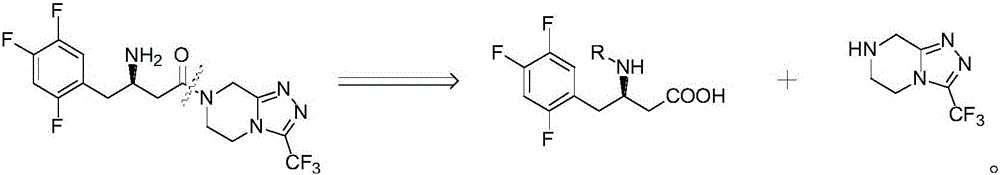
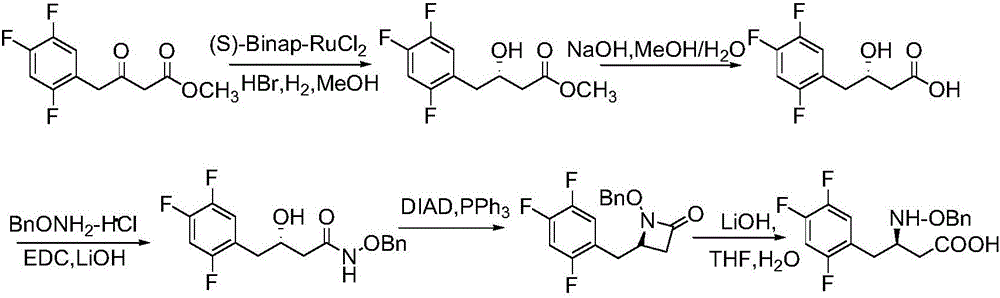
Preparation method of Sitagliptin midbody of beta-amino acid
A sitagliptin and amino acid technology, applied in the field of preparation of intermediate β-amino acids, can solve the problems of reduced atom economy, unusable, serious environmental pollution, etc., achieve good industrial application and economic value, low cost, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 compound 3
[0053] Dissolve 1.74g (10mmol) of compound 2 (2,4,5-trifluorophenylacetaldehyde, the same below) and 1.66g (11mmol) of L-amphetamine alcohol in 50mL of dichloromethane, and add di Magnesium iodide 278mg (1.0mmol), after stirring for 10 minutes, add dropwise 4.0g (12mmol) tributylallyl stannane, react at 20°C, TLC detects that the reaction is over, add Na 2 S 2 o 3 The aqueous solution was used to quench the reaction, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain 2.86 g of compound (3), with a yield of 82%.
Embodiment 2
[0054] The preparation of embodiment 2 compound 3
[0055] Dissolve 1.4g (8mmol) of compound 2 and 1.33g (8.8mmol) of L-amphetamine in 40mL of dichloromethane, add 145mg (0.8mmol) of magnesium dibromide at 20°C under nitrogen protection, stir for 10 minutes and then add dropwise 3.2 g (9.6 mmol) tributylallylstannane. React at 20°C, TLC detects the end of the reaction, add Na 2 S 2 o 3 The aqueous solution was used to quench the reaction, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain 2.1 g of compound (3) with a yield of 75%.
Embodiment 3
[0056] The preparation of embodiment 3 compound 3
[0057] 3.48 g (20 mmol) of compound 2 and 3.32 g (22 mmol) of L-amphetamine were dissolved in 100 mL of dichloromethane. Magnesium perchlorate 246mg (2.0mmol) was added at 20°C under nitrogen protection. After stirring for 10 minutes, 8.0 g (24 mmol) of tributylallylstannane was added dropwise, reacted at 20° C., and TLC detected that the reaction was completed, and Na 2 S 2 o 3 The aqueous solution was used to quench the reaction, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain 4.8 g of compound (3) with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com