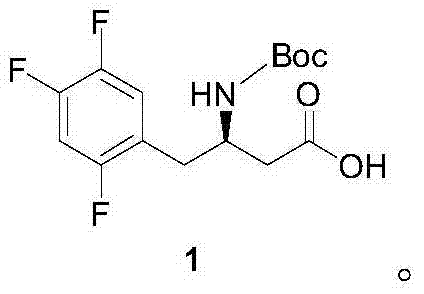
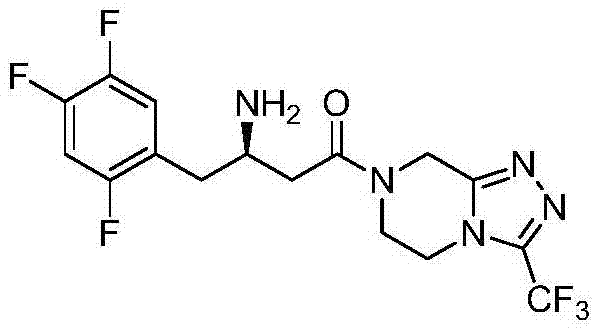
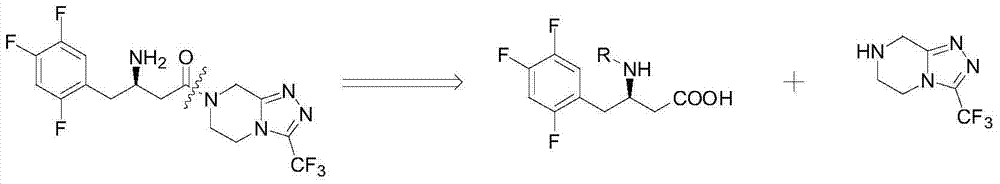
Preparation method of sitagliptin intermediate
A reaction time, amino acid technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carbamate derivatives, etc., can solve the problems of reducing atomic economy, serious environmental pollution, unavailability and other problems, and achieve good industrial application. and economic value, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 compound enamine 3
[0059] 3.0 g of Compound 2 was dissolved in methanol, 4.12 g of R-(+)-α-phenethylamine was added, and the reaction was stirred at room temperature for 24 h. Concentrate under reduced pressure to remove methanol, add a small amount of water, extract with ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and purify by column chromatography to obtain 3.92 g of colorless oil 3 with a yield of 92%.
Embodiment 2
[0060] The preparation of embodiment 2 compound enamine 3
[0061] 3.0 g of compound 2 was dissolved in methanol, 4 g of D-(-)-phenylglycinol was added, and the reaction was stirred at room temperature for 24 h. Concentrate under reduced pressure to remove methanol, add a small amount of water, extract with ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and purify by column chromatography to obtain 4.0 g of a colorless oil with a yield of 90%.
Embodiment 3
[0062] The preparation of embodiment 3 compound 4
[0063] Add 2.4 g of sodium cyanoborohydride into 30 mL of tetrahydrofuran, then add 24 mL of acetic acid, add 3.0 g of compound 3 prepared in Example 1 under ice-cooling, slowly warm to room temperature, and stir overnight. Adjust the pH value to 7.0 with saturated sodium carbonate solution, extract with ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and separate by column chromatography (petroleum ether-ethyl acetate, volume ratio 2:1) to obtain Colorless oil (5) 2.51g, yield 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com