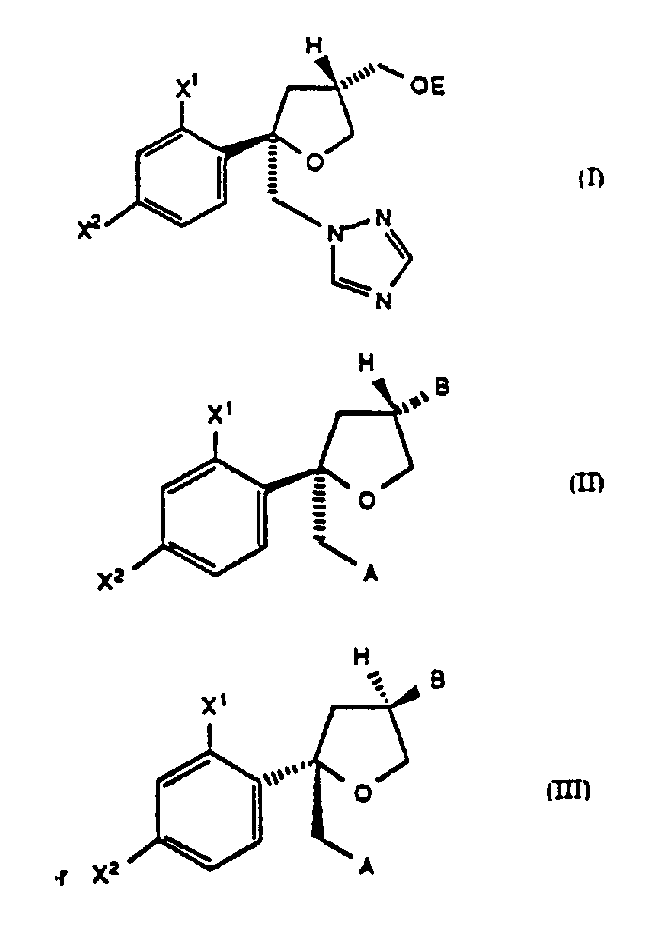
Process for preparing intermediates for synthesis of antifungal agents
A compound and chiral compound technology, applied in bulk chemical production, organic chemistry, fermentation, etc., can solve the problems of high cost and low efficiency of prior art methods for tosylate intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0117] (4S)-(-)-4-Isopropyl-2-oxazolidinone (400 mg, 3.1 mmol) was dissolved in 4 ml THF and cooled to -78°C. 2ml (3.2mmol) of 1.6M n-butyllithium in hexane was added and the mixture was stirred at -78°C for 10 minutes to give a solution of the title oxazolidinone salt.
preparation example 2
[0119] step (a)
[0120] Allyl alcohol (6.25g, 31.53mmol), triethyl orthoacetate (20.46g, 126.12mmol) and 5 drops of propionic acid were mixed, the mixture was heated at 120°C, and 4ml of EtOH was collected by distillation. Heating was continued and excess triethyl orthoacetate (14ml) was distilled off to give a residue. The residue was mixed with KOH (3.5 g, 63 mmol), 16 ml MeOH and 4 ml water and stirred at room temperature overnight (@18 hours). Dilute the mixture with water and wash with cold CH 2 Cl 2 After washing, the aqueous layer was acidified to pH=3 by adding 0.1M HCl. Extract with 3 portions of EtOAc, combine the EtOAc extracts, wash with Na 2 SO 4 Drying and concentration afforded 6.75 g of the acid product. MS=213(M+H) + . step (b)
[0121] The acid product from step (a) (0.5 g, 2.36 mmol), KOH (0.13 g, 2.36 mmol) and 5 ml EtOH were combined and stirred at room temperature for 2 hours. Evaporation of the solvent gave a residue which was dissolved in...
preparation example 3
[0124] Allyl alcohol (5.37g, 31.58mmol) was dissolved in 50ml CH 2 Cl 2 and cool the resulting solution to 0-5 °C. Join PBr 3 (1.0 mL, 10.53 mmol), warmed to room temperature and stirred for 1 h while monitoring the reaction by TLC (silica gel, 25% EtOAc / hexanes). Add 50 ml of ice water, stir for 5 min, separate the layers and wash with MgSO 4 Dry the organic layer. Concentration in vacuo afforded 6.45 g of bromide product. MS=233M + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com