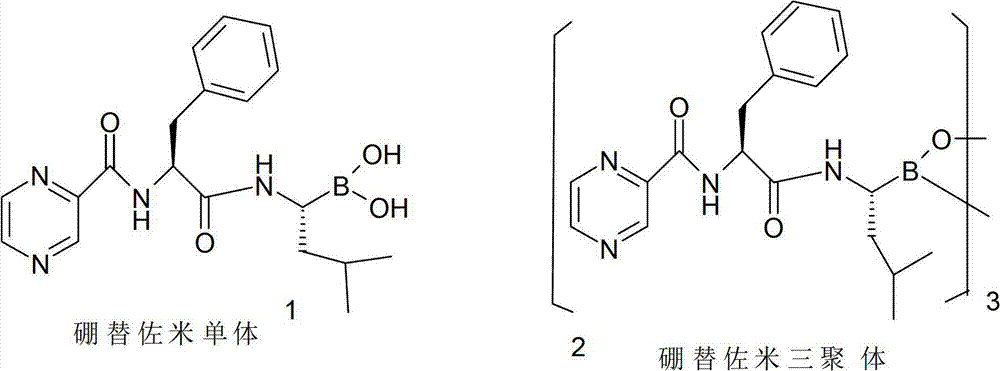
Method for preparing bortezomib with (one)-cypress camphor serving as chiral auxiliary reagent
A chiral adjuvant, bortezomib technology, applied in the production of bulk chemicals, peptides, etc., can solve the problems of difficult recovery, expensive pinanediol, high production cost, etc., to reduce production costs and facilitate The effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
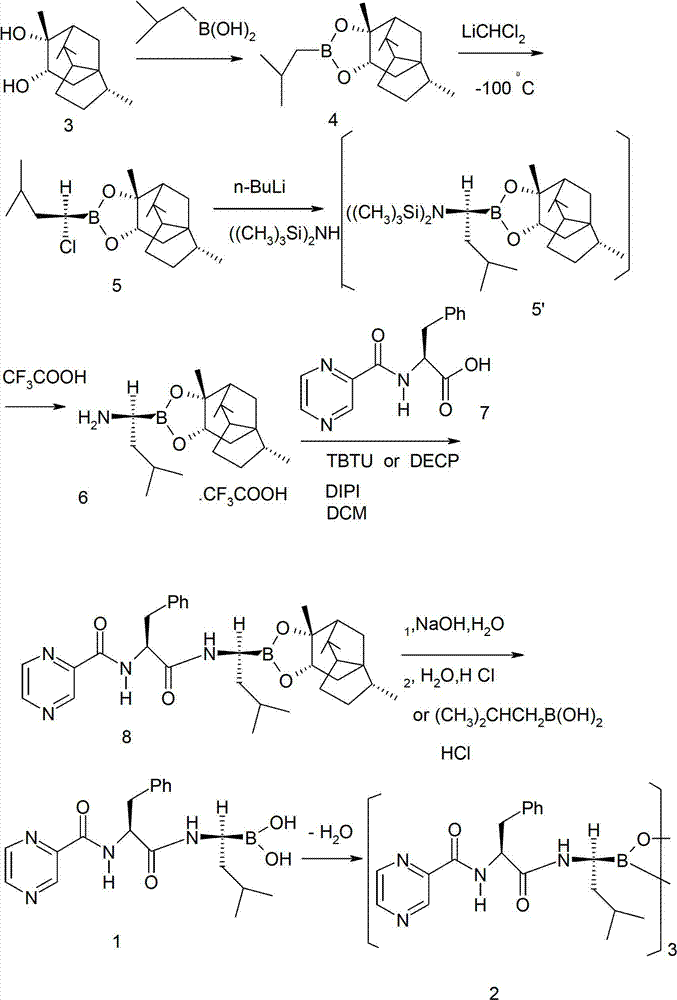
[0023] Add (-)-cedarediol (24g, 100mmol) and 200ml of petroleum ether (60-90°C) into the reaction flask, and dissolve under stirring at room temperature. Then add isobutylboronic acid (100mmol), and stir the reaction at room temperature for 2-4 hours, separate the water layer, dry the organic layer with anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and perform silica gel column chromatography (petroleum ether). elution) to obtain 30 g of isobutylboronic acid-(-)-cedarediol ester (4) as an oil. The yield is 84%.
Embodiment 2
[0025] Add (-)-cedarediol (24g, 100mmol) and 200ml of dichloromethane into the reaction flask, and dissolve under normal temperature stirring. Then add isobutylboronic acid (100mmol), and stir the reaction at room temperature for 2-4 hours, separate the water layer, dry the organic layer with anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and perform silica gel column chromatography (petroleum ether). elution) to obtain 28 g of isobutylboronic acid-(-)-cedarediol ester (4) as an oil. Yield 79%.
Embodiment 3
[0027]Add (-)-cedarediol (24g, 100mmol) and 200ml of toluene into the reaction flask, and dissolve with stirring at room temperature. Then add isobutylboronic acid (100mmol), and stir the reaction at room temperature for 2-4 hours, separate the water layer, dry the organic layer with anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and perform silica gel column chromatography (petroleum ether). elution) to obtain 31 g of isobutylboronic acid-(-)-cedarediol ester (4) as an oil. The yield is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com