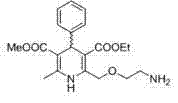
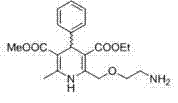
Method for producing S-(-)-amlodipine besylate
A kind of technology of levamlodipine besylate and production method, which is applied in the field of drug synthesis, can solve the problems of high price, high production cost, high production cost, etc., achieve the effect of good yield and crystal form, and good industrial application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of levamlodipine besylate
[0027] Step 1) Preparation of S-(-)-amlodipine-L-tartrate from (R,S)-amlodipine
[0028] Mix 1050ml of N,N-dimethylformamide and 150ml of water evenly, filter and set aside to obtain 1L resolution aid solution. Weigh 100g of racemic amlodipine and dissolve in 900ml of the above mixed solution, filter to remove insoluble impurities, add the filtrate to a clean 2L three-neck flask and stir at room temperature for 30 minutes. Weigh 18.4g L-tartaric acid and dissolve it in 300ml resolution solution, filter and add dropwise into the reaction solution, and stir at room temperature for 50 minutes. Add a small amount of S-(-)-amlodipine-L-tartrate seed crystals, and continue stirring for 4 hours to crystallize. Filtrate under reduced pressure to obtain a white solid, wash with 150 ml of cold acetone, and dry under pressure for 10 hours to obtain 50.4 g of S-(-)-amlodipine-L-tartrate.
[0029] Mp: 135-138 ℃, MS (Cl): (M+H)...
Embodiment 2
[0033] Example 2 Preparation of levamlodipine besylate
[0034] Step 1) Preparation of S-(-)-amlodipine-L-tartrate from (R,S)-amlodipine
[0035]Mix 600ml of N,N-dimethylformamide and 600ml of water evenly, filter and set aside to obtain 1L resolution aid solution. Weigh 100g of racemic amlodipine and dissolve in 900ml of the above mixed solution, filter to remove insoluble impurities, add the filtrate to a clean 2L three-neck flask and stir at room temperature for 30 minutes. Weigh 36.65g L-tartaric acid and dissolve it in 300ml resolution solution, filter and add dropwise to the reaction solution, and stir at room temperature for 50 minutes. Add a small amount of S-(-)-amlodipine-L-tartrate seed crystals, and continue stirring for 4 hours to crystallize. Filtrate under reduced pressure to obtain a white solid, wash with 150 ml of cold acetone, and dry under pressure for 10 hours to obtain 40.8 g of S-(-)-amlodipine-L-tartrate.
[0036] Mp: 135-138 ℃, MS (Cl): (M+H) / z:...
Embodiment 3
[0040] Example 3 Preparation of levamlodipine besylate
[0041] Step 1) Preparation of S-(-)-amlodipine-L-tartrate from (R,S)-amlodipine
[0042] Mix 1090ml of N,N-dimethylformamide and 109ml of water evenly, filter and set aside to obtain 1L resolution aid solution. Weigh 100g of racemic amlodipine and dissolve in 900ml of the above mixed solution, filter to remove insoluble impurities, add the filtrate to a clean 2L three-neck flask and stir at room temperature for 30 minutes. Weigh 7.33g L-tartaric acid and dissolve it in 300ml resolution solution, filter and add dropwise to the reaction solution, and stir at room temperature for 50 minutes. Add a small amount of S-(-)-amlodipine-L-tartrate seed crystals, and continue stirring for 4 hours to crystallize. Filtrate under reduced pressure to obtain a white solid, wash with 150 ml of cold acetone, and dry under pressure for 10 hours to obtain 53.6 g of S-(-)-amlodipine-L-tartrate.
[0043] Mp: 135-138 ℃, MS (Cl): (M+H) / z...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com