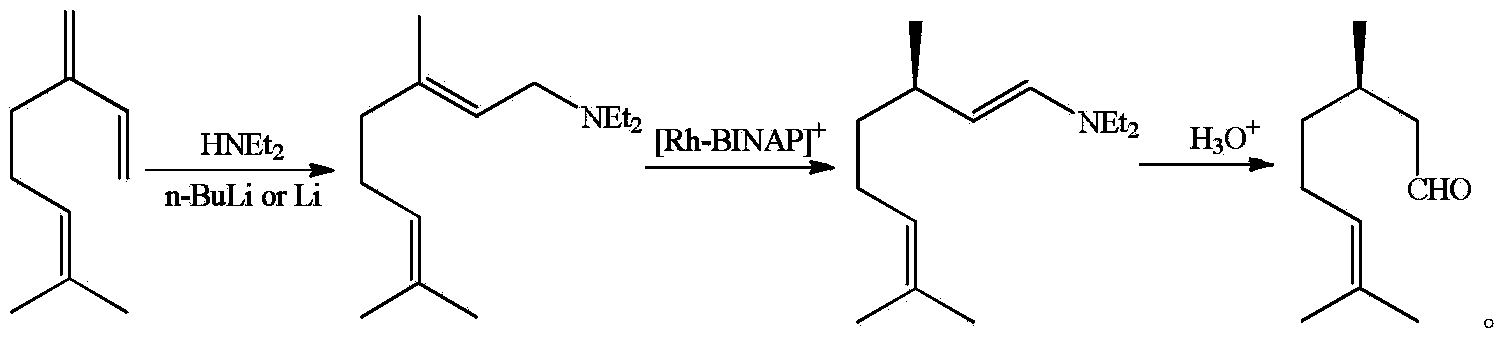
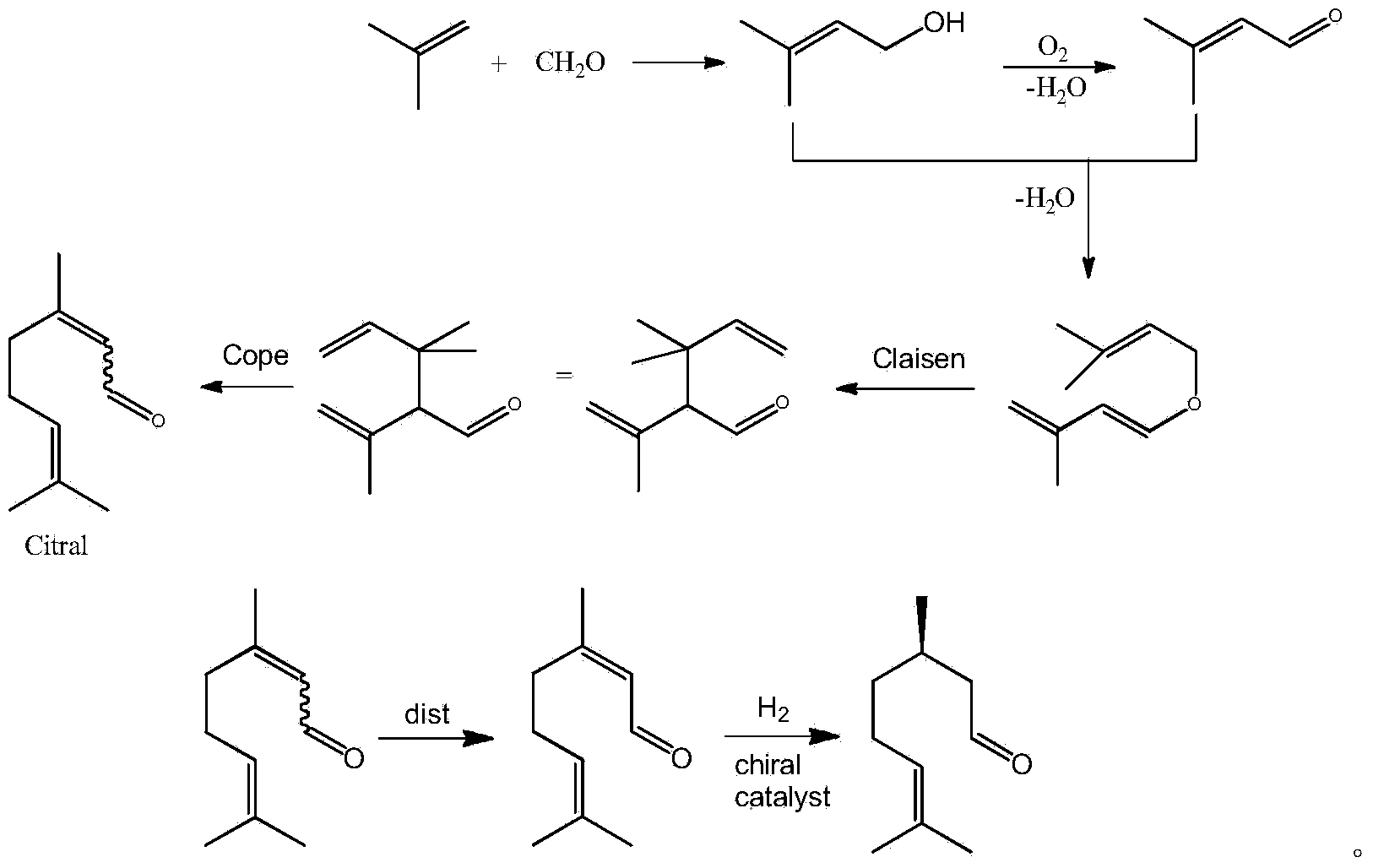
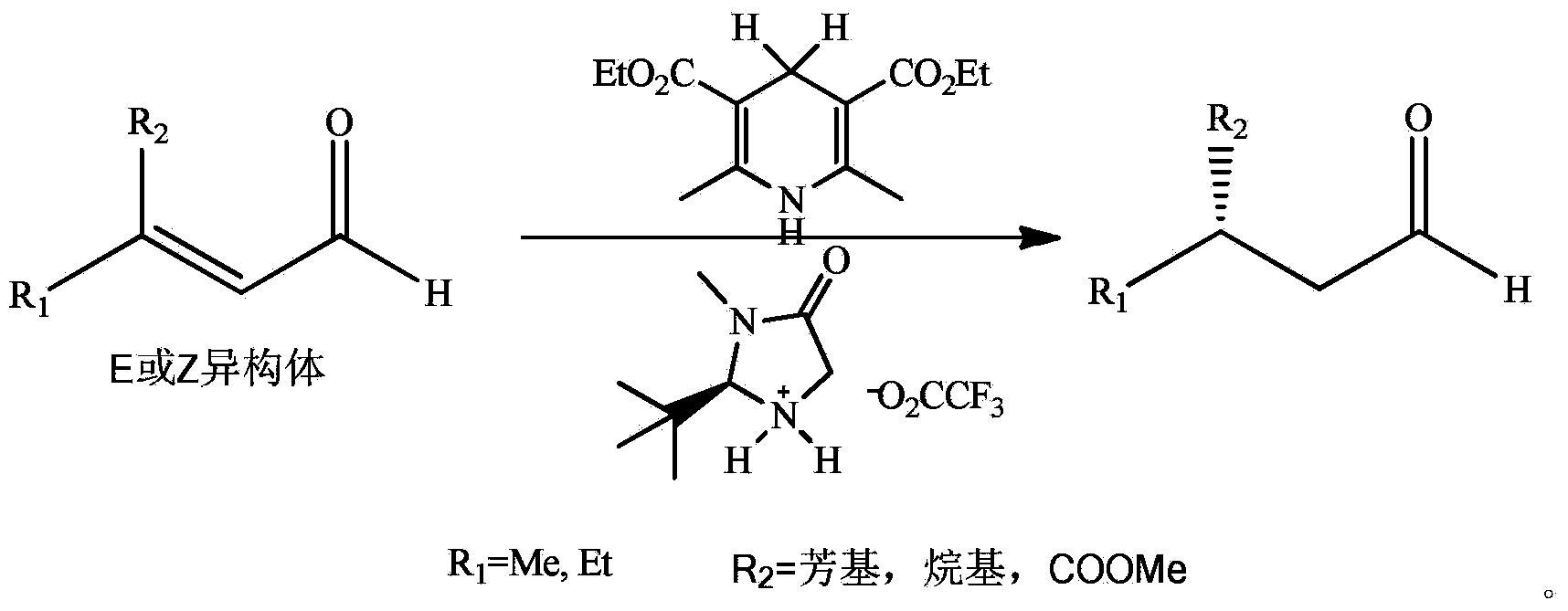
Asymmetric synthesis method of dextral citronellal
An asymmetric, citronellal technology, applied in the field of asymmetric synthesis of organic chemistry, can solve the problems of difficult reuse of trans-citral, high production equipment requirements, etc., and achieve the effects of simple catalyst preparation, mild reaction conditions, and convenient recovery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Take a 250ml three-necked flask, and add citral (15.2g, 0.1mol), (R)-2-[bis(phenyl)]methyltetrahydropyrrole (24mg, 0.1mmol) to it successively under nitrogen protection, Trifluoroacetic acid (12 mg, 0.1 mmol), 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate diethyl ester (75.9 g, 0.3 mol), and 80 ml of benzene was added to dissolve the solid . Stir at room temperature for 10 h, remove the solvent under reduced pressure, and purify by column chromatography to obtain 13.9 g of (R)-citronellal light yellow liquid with a yield of 90% and an ee value of 80%.
Embodiment 2
[0033] Take a 250ml three-necked flask, add citral (15.2g, 0.1mol) and (R)-2-[bis(4-methylphenyl)]methyltetrahydropyrrole (27mg, 0.1mmol), trichloroacetic acid (16mg, 0.1mmol), 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate diethyl ester (75.9g, 0.3mol), and add 80ml THF dissolved the solid. Stir at -20°C for 20 h, remove the solvent under reduced pressure, and purify by column chromatography to obtain 13.5 g of (R)-citronellal light yellow liquid with a yield of 88% and an ee value of 80%.
Embodiment 3
[0035] Take a 250ml three-necked flask, add citral (15.2g, 0.1mol), (R)-2-[bis(4-tert-butylphenyl)]methyltetrahydropyrrole (35mg , 0.1mmol), trifluoroacetic acid (12mg, 0.1mmol), 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate diethyl ester (25.3g, 0.1mol), and Add 80 mL of tetrahydrofuran to dissolve the solid. Stir at 30°C for 24 h, remove the solvent under reduced pressure, and purify by column chromatography to obtain 13.6 g of (R)-citronellal light yellow liquid with a yield of 88% and an ee value of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com