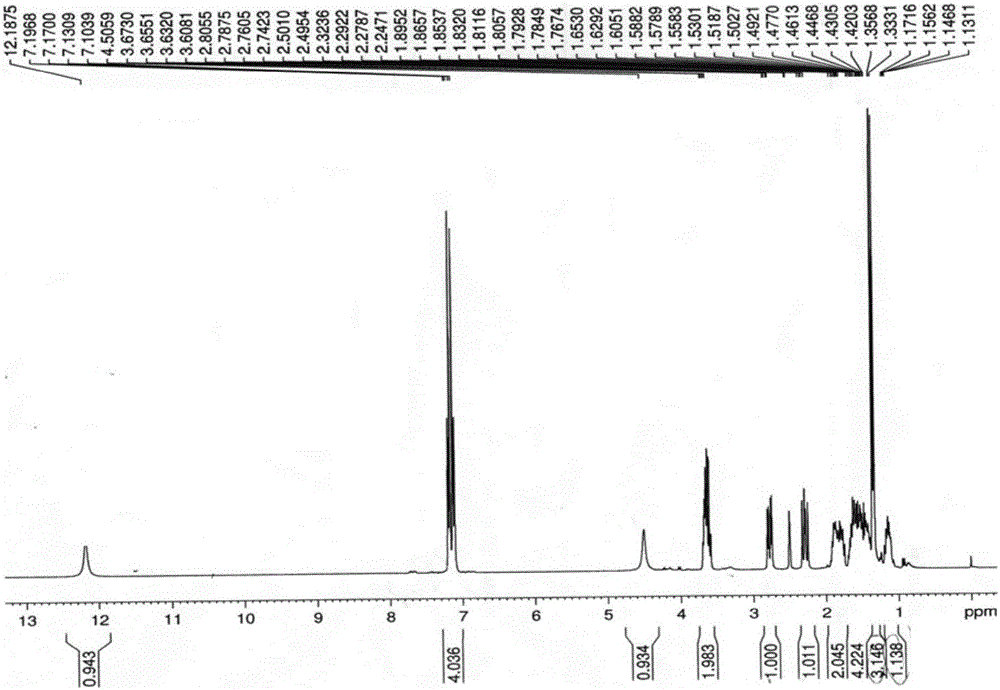
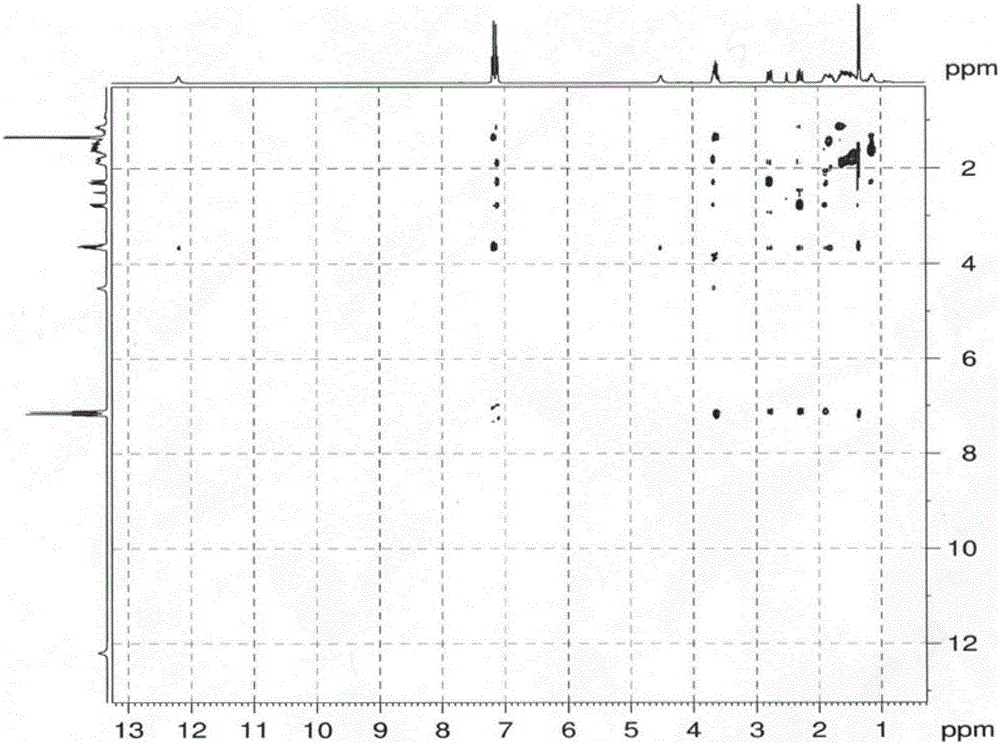
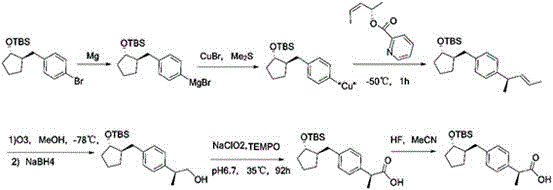
Method for preparing loxoprofen active metabolite
A technology of compounds and chiral auxiliary agents, which is applied in the field of synthesis of trans-hydroxyl active metabolites, can solve the problems of energy consumption that is not suitable for large-scale production, difficult to obtain reagents, harsh conditions, etc., and achieves easy operation and avoids purification. Steps, high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of embodiment 1 formula 2 compound
[0046] Put 1000g of the compound of formula 1 into 7000mL of ethyl acetate and stir to dissolve it. Slowly add 240g of S-methylbenzylamine dropwise at room temperature. Solids appear continuously during the dropwise addition. Continue to stir for 1h after the dropwise addition, and obtain about 650g of the crude product by suction filtration; Put the crude product into 6500mL toluene, raise the temperature to 60-65°C, slowly add about 260-325mL of methanol under stirring, stir until completely dissolved, then slowly lower to 5-10°C, continue to stir for 2h, filter with suction, and dry the filter cake to obtain a solid 570g, the solid was recrystallized once with the toluene methanol system of the above ratio, and the obtained solid was put into 2000mL of purified water and stirred evenly, and 10% dilute phosphoric acid was added to adjust the pH to 2-3, extracted with 1500mL of ethyl acetate, and the compound of formula 2 i...
Embodiment 2
[0047] Synthesis of embodiment 2 formula 3 compound
[0048] Dissolve about 300g of the solid compound of formula 2 in 4500mL of methanol, add 150g of concentrated sulfuric acid dropwise at 0-5°C, after the drop is complete, the reaction is complete at room temperature, add 200mL of ethyl acetate and 200mL of pure water, stir, let stand and separate, and the organic layer After washing, drying and concentration, about 290 g of the compound of formula 3 was obtained as a yellow oil.
Embodiment 3
[0049] Synthesis of embodiment 3 formula 5 compound
[0050] Put 135g of L-phenylalaninol and 400g of triethylamine into a mixture of 600ml of dioxane and 400ml of pure water, and drop 200g of di-tert-butyl dicarbonate in 100mL of dioxane at 0-5°C The solution, dropwise, complete reaction at room temperature, add 400mL of dichloromethane, 200mL of pure water and stir, let it stand for stratification, wash and dry the organic phase with citric acid water, saturated sodium bicarbonate solution, pure water, and saturated brine, and concentrate to obtain The compound of formula 5 is about 205g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com