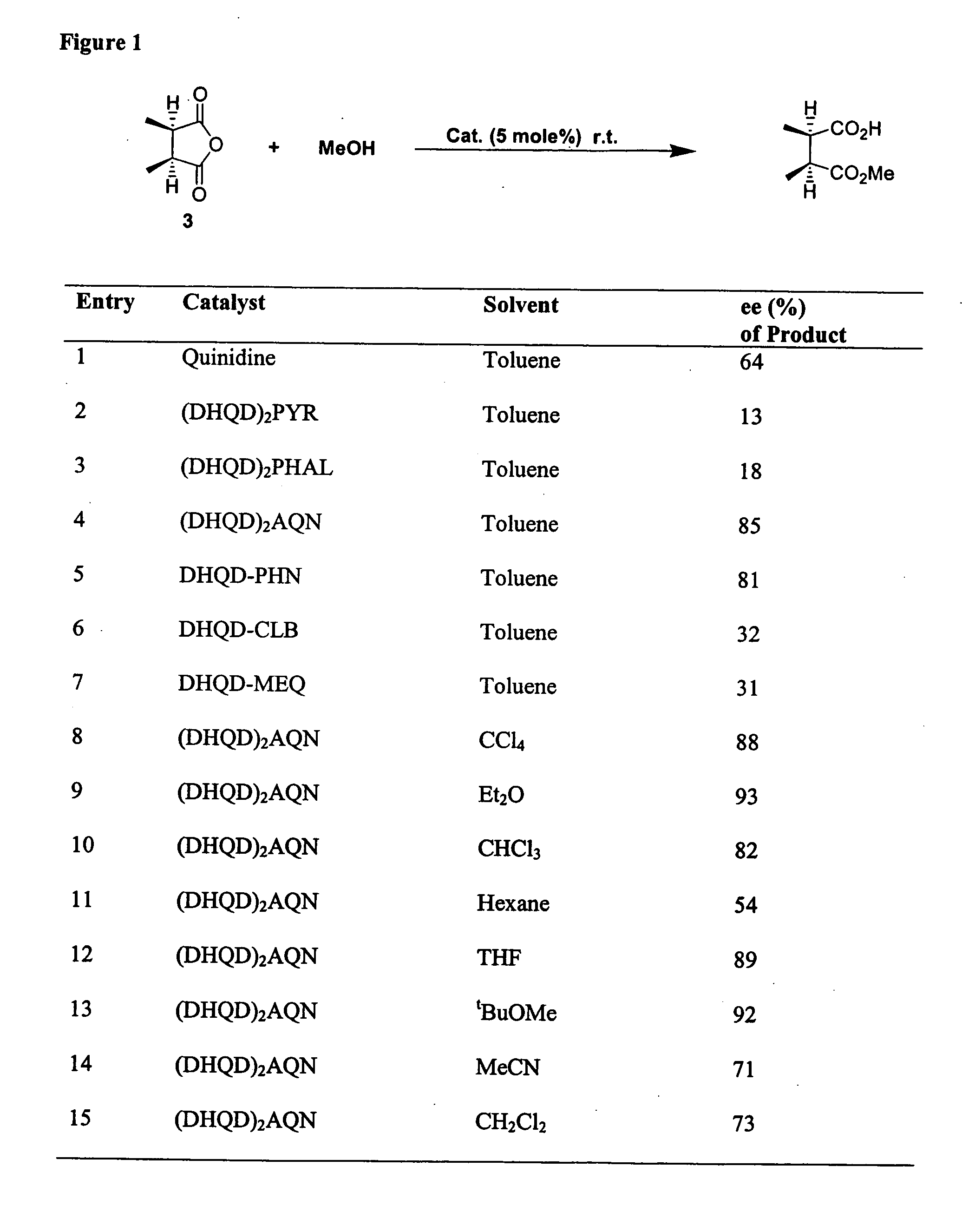
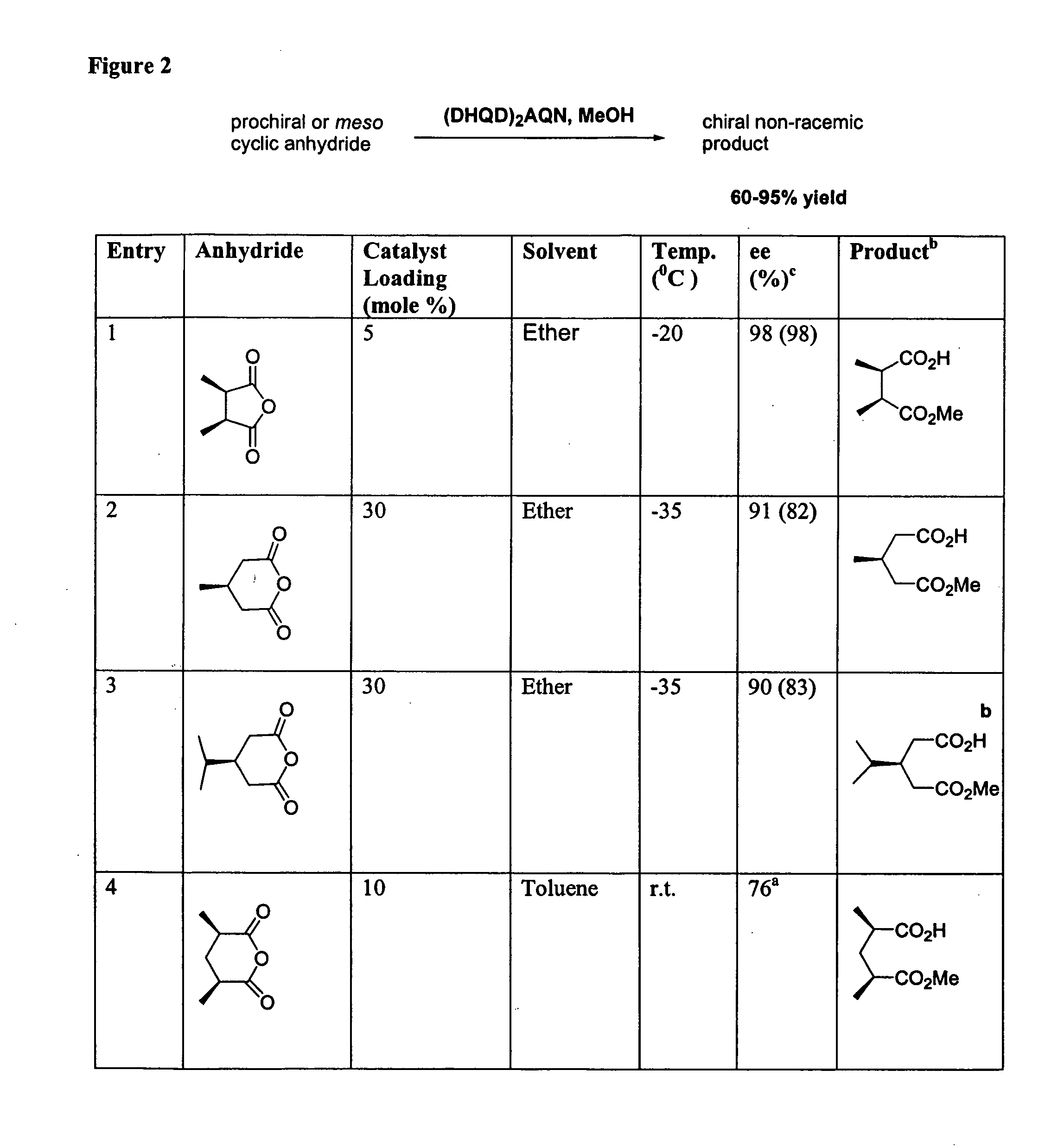
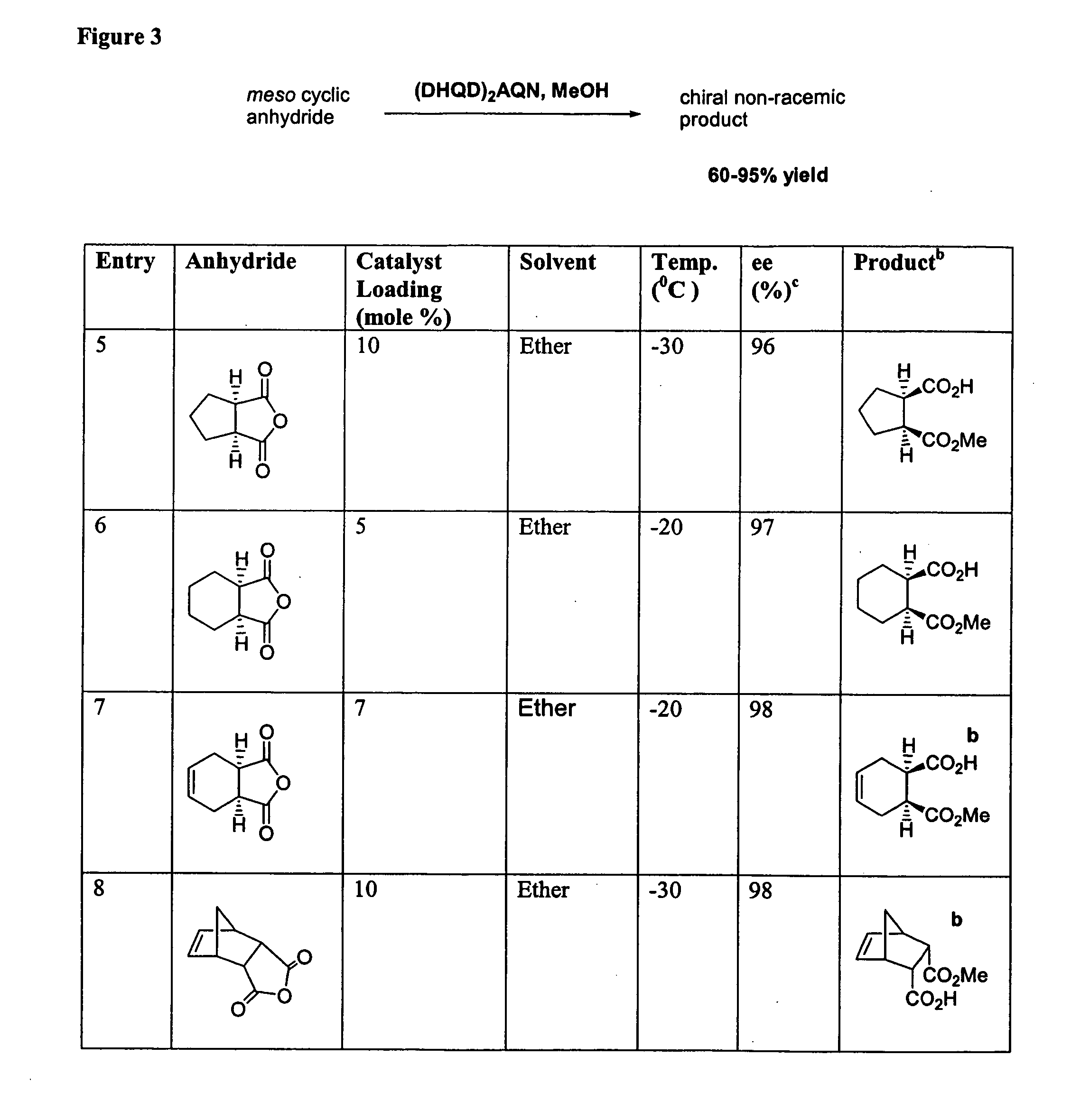
Catalytic asymmetric desymmetrization of prochiral and meso compounds
a technology of prochiral and meso compounds, applied in the preparation of carboxylic acid esters, chemistry apparatus and processes, organic chemistry, etc., can solve the problems of wasting half of the material, reducing efficiency, and wasting tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
REFERENCES AND NOTES FOR EXAMPLE 1
[0132] 1. Toyota, M.; Yokota, M.; Ihara, M. Organic Lett. 1999, 1, 1627-1629. [0133] 2. Couche, E.; Deschatrettes, R.; Poumellec, K.; Bortolussi, M.; Mandvile, G.; Bloch, R. Synlett. 1999, 87-88. [0134] 03. Paterson, I.; Cowden, C. J.; Woodrow, M. D. Tetrahedron Lett. 1998, 39, 6037-6040. [0135] 4. a) Borzilleri, R. B.; Weinreb, S. M. J. Am. Chem. Soc. 1994, 116, 9789-9790. [0136] b) Borzilleri, R. B.; Weinreb, S. M.; Parvez, M. J. Am. Chem. Soc. 1995, 117, 10905-10913. [0137] 5. Marie, F. B. C.; Mackiewicz, P.; Roul, J. M.; Buendia, J. Tetrahedron Lett. 1992, 33, 4889-4892. [0138] 6. a) Ohtani, M.; Matsuura, T.; Watanabe, F.; Narisada, M. J Org. Chem. 1991, 56, 4120-4123. b) Ohtani, M.; Matsuura, T.; Watanabe, F.; Narisada, M. J Org. Chem. 1991, 56, 2122-2127. [0139] 7. Wender, P. A.; Singh, S. K. Tetrahedron Lett. 1990, 31, 2517-1520. [0140] 8. Suzuki, T.; Tomino, A.; Matsuda, Y.; Unno, K.; Kametani, T. Heterocycles, 1980, 14, 1735-1738. [0141] 9....
example 2
General Method for Synthesizing Tertiary Amine Catalysts
[0150]
[0151] To a solution of diamine 1 (1.40 g, 4.67 mmol) in dry tetrahydrofuran (93 mL) under nitrogen at room temperature was added sodium hydride (60% suspension in mineral oil, 1.87 g, 46.7 mmol). The mixture was stirred for 10 minutes, and then glycidol nosylate 2 was added. After being stirred for 88 hours, the mixture was filtered, and the filtrate was concentrated under reduced pressure. The resulting residue was purified by chromatography [basic aluminum oxide, CH3OH:CH2Cl2 (1:100 to 1:20)] to give the chiral tertiary amine 3 (667 mg, 35%) as a white solid.
example 3
Catalytic Desymmetrization of a Meso Bicyclic Succinic Anhydride Comprising a Urea
[0152]
[0153] To a mixture of anhydride (16.8 mg, 0.05 mmol) and DHQD-PHN (20 mol %, 5 mg) in Et2O (2.5 mL) at —40° C., anhydrous MeOH (0.5 mmol, 20.2 ul) cooled at —20° C. was added in one portion. The resulting mixture was stirred until the reaction was complete (˜30 hrs) as monitored by TLC (20% MeOH in CH2Cl2). The reaction was quenched with aqueous HCl (1 N, 3 mL). The aqueous layer was extracted with EtOAc (2×10 mL). The combined organic layer was dried over MgSO4 and concentrated. The residue was purified by flash chromatography (100% CH2Cl2 to 10% MeOH in CH2Cl2) to afford the hemiester (16.7 mg, 91% yield). The ee of the hemiester was determined to be 93% by converting the hemiester into the corresponding ester amide (J. Chem. Soc. Perkin. Trans l 1987, 1053) via a reaction of the hemiester with (R)- 1-(1-napthyl) ethyl amine. The ester amide was analyzed by chiral HPLC (Chiralpak, OD, 280 nm,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com