A kind of synthetic method of rosuvastatin calcium chiral isomer impurity
A technology of rosuvastatin calcium and chiral isomers, applied in organic chemistry methods, organic chemistry and other directions, can solve problems such as failure to prepare successfully, difficult to purify, complicated reactions, etc., and achieves easy operation, short route, purity and high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
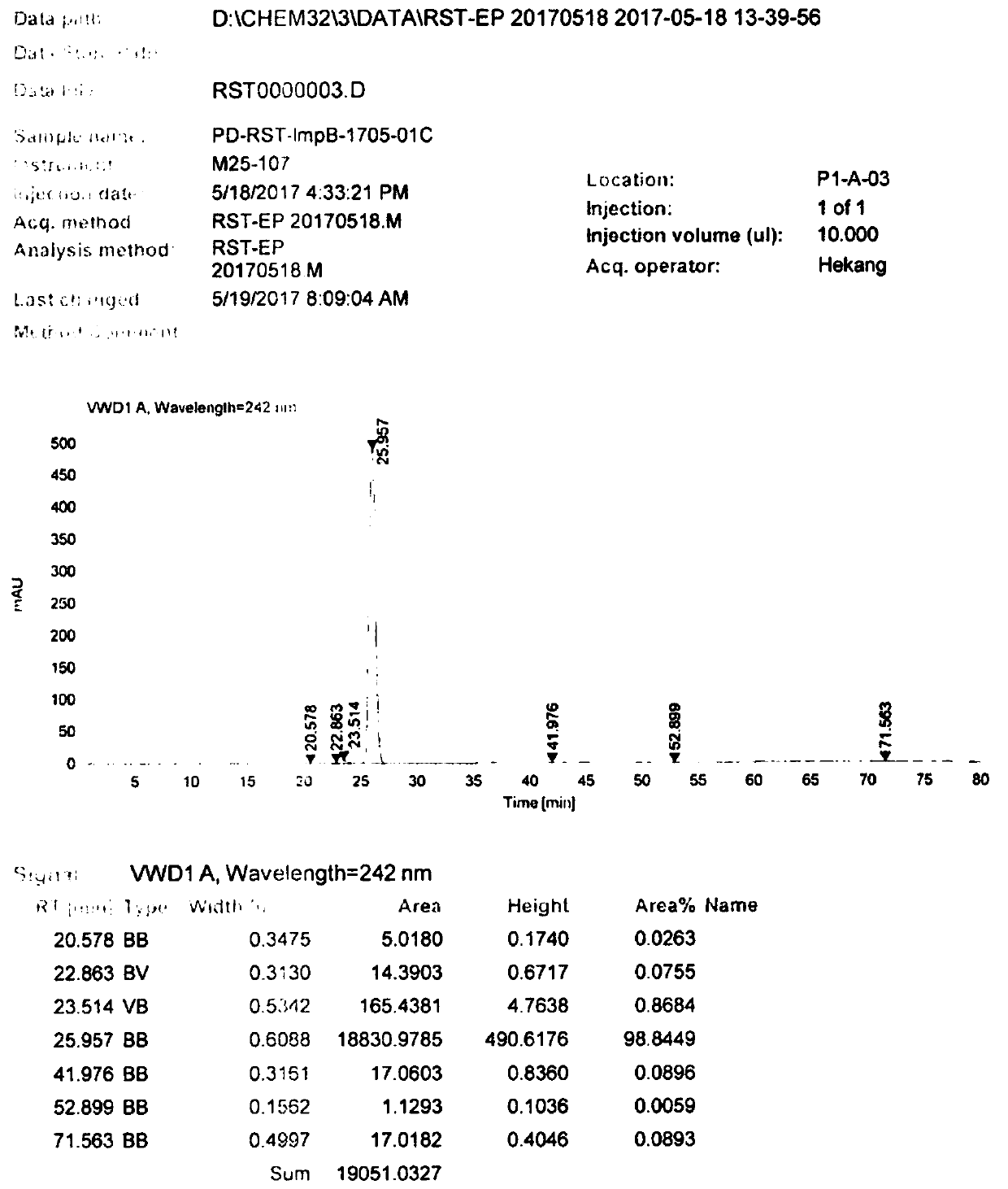
Image
Examples
Embodiment 1
[0034] The synthetic method of the rosuvastatin calcium chiral isomer impurity of the present embodiment comprises the following steps:
[0035] (1) Add 20g of compound I to a 500mL three-necked reaction flask, add 220mL of acetonitrile and stir to dissolve, then add 60mL of 0.05M hydrochloric acid dropwise, after the dropwise addition, the reaction solution is kept at 35-40°C for 3 hours until the raw materials disappear (TLC: ethyl acetate Esters: petroleum ether = 6:1), adjust the pH value to neutral with 5% sodium bicarbonate solution, remove acetonitrile by distillation under reduced pressure, add dichloromethane to extract twice (100mL*2), dry over anhydrous sodium sulfate, filter , the filtrate was transferred to a 500mL three-necked reaction flask, 60g of manganese dioxide was added, and the reaction was carried out under reflux for 20 hours. After the reaction was completed, it was filtered to obtain the filtrate, which was distilled under reduced pressure and concentr...
Embodiment 2
[0040] The synthetic method of the rosuvastatin calcium chiral isomer impurity of the present embodiment comprises the following steps:
[0041] (1) Add 2g of compound I in a 50mL round-bottomed flask, add 20mL of acetonitrile and stir to dissolve, add dropwise 10mL of 0.05M hydrochloric acid, after dripping, the reaction solution is incubated at room temperature for reaction, and the reaction process is monitored by TLC, (TLC: ethyl acetate: Petroleum ether=6:1); After the reaction is complete, adjust the pH of the reaction solution to neutral, distill off ethanol under reduced pressure, add dichloromethane to extract twice (20mL*2), dry over anhydrous sodium sulfate, filter, and transfer the filtrate to Add 10g of manganese dioxide to a 100mL three-necked reaction flask, reflux and stir until the reaction is complete. After the reaction, filter to obtain the filtrate, distill under reduced pressure, and concentrate to dryness. The obtained light yellow oil is compound III, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com