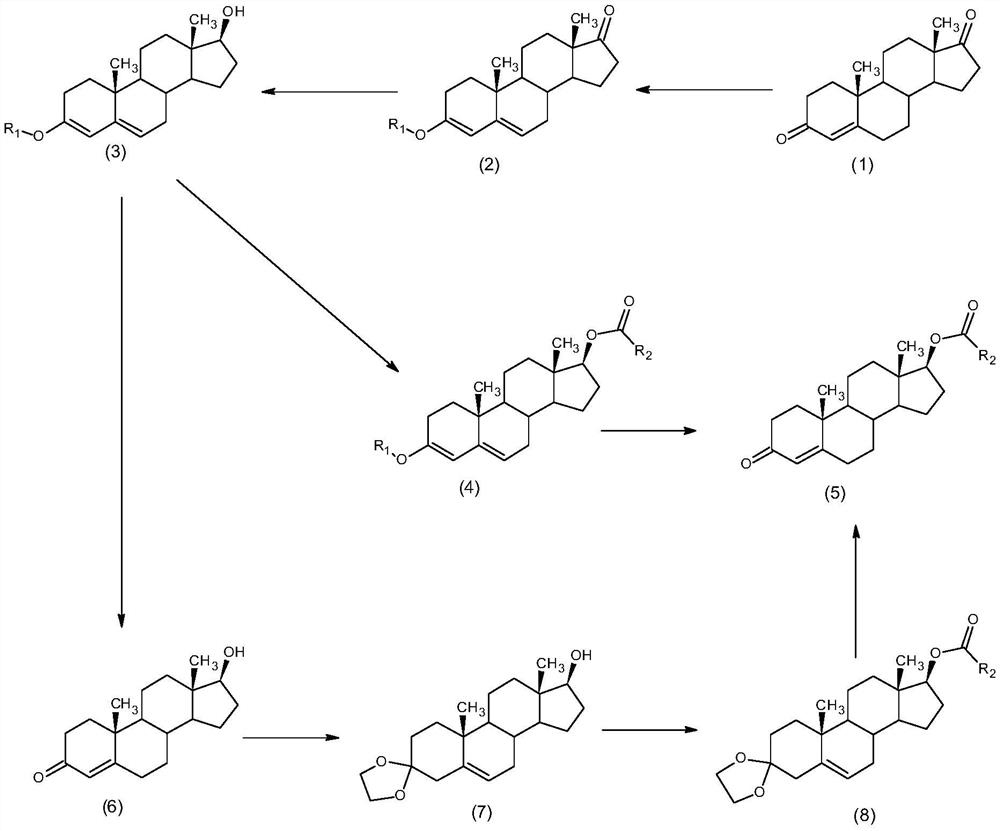
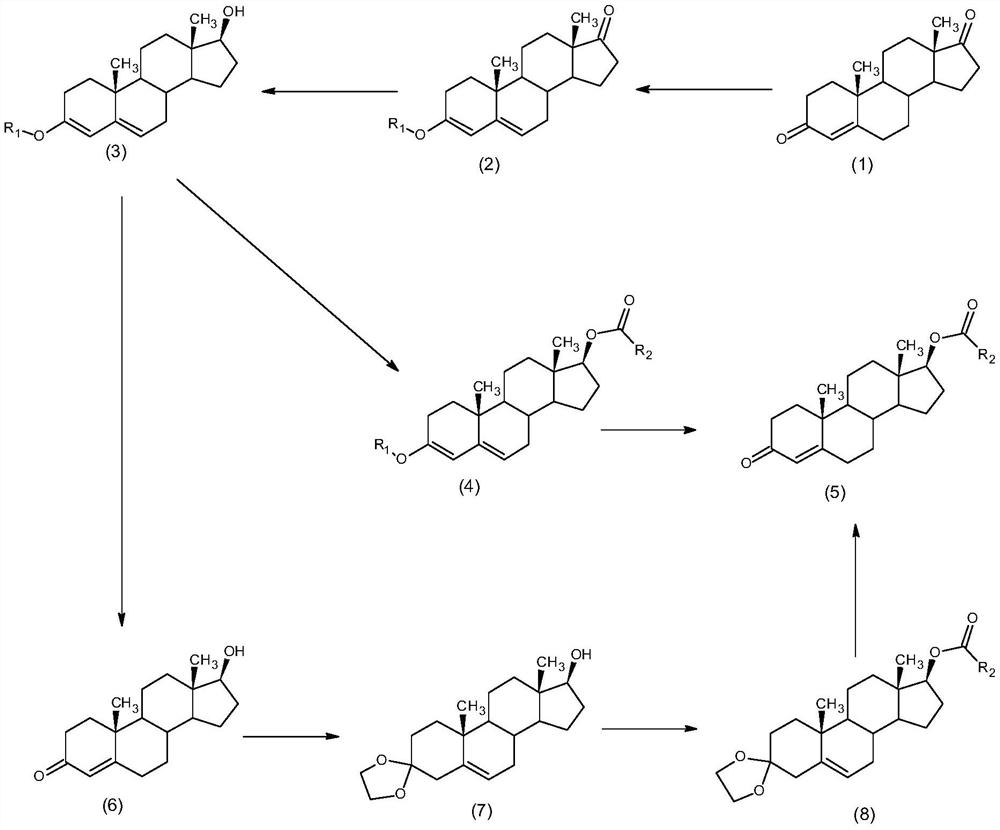
Synthesis method of alkyl acid testosterone
A synthesis method and technology of alkyl acid, applied in the field of synthesis of alkyl acid testosterone, can solve the problems of large environmental pollution, many by-products, low yield, etc., and achieve the effects of low price, stable market supply, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of embodiment 1 testosterone propionate
[0034] 1) Under the protection of nitrogen, put 10g of 4-androstenedione into 5ml of anhydrous methanol, add 3.82ml of trimethyl orthoformate, 0.1g of pyridinium p-toluenesulfonate, and keep warm at 20°C for reaction. After the reaction is completed, add 0.1ml of triethylamine was concentrated and filtered to obtain 10.2g of 3-methoxy-androst-3,5-dien-17-one.
[0035] 2) Put 10.2g of 3-methoxy-androst-3,5-dien-17-one into 10.2ml of methanol, add 0.1ml of pyridine, 1g of sodium borohydride, keep warm at 40°C, after the reaction is completed, water analysis, filtration, and drying to obtain 10.1g of 17β-hydroxyl-3-methoxyl-androst-3,5-diene
[0036] 3) Under nitrogen protection, put 10.1g of 17β-hydroxy-3-methoxy-androst-3,5-diene into 101ml of dichloromethane, add 0.01g of 4-dimethylaminopyridine, 5g of N,N - Diisopropylcarbodiimide (DIC), start stirring, add 12.1g propionic acid, keep warm at 5°C until complete r...
Embodiment 2
[0037] The synthesis of embodiment 2 testosterone enanthate
[0038] 1) Under the protection of argon, put 10g of 4-androstenedione into 100ml of absolute ethanol, add 17.4ml of triethyl orthoformate, 0.5g of pyridine hydrochloride, and keep warm at 40°C for reaction. After the reaction is completed, add 0.5 ml of triethylamine, concentrated and filtered to obtain 10.5 g of 3-ethoxy-androst-3,5-dien-17-one.
[0039] 2) Put 10.5g of 3-ethoxy-androst-3,5-dien-17-one into 52.5ml of ethanol, add 31.5ml of pyridine, 10.5g of potassium borohydride, and keep warm at 60°C. After the reaction is completed, Water analysis, filtration, and drying to obtain 10.4 g of 17β-hydroxy-3-ethoxy-androsta-3,5-diene
[0040] 3) Put 10.4g of 17β-hydroxy-3-ethoxy-androst-3,5-diene into 52ml of tetrahydrofuran, add 10.4ml of 20% dilute hydrochloric acid, keep warm at 20-30°C, after the reaction is completed, oxidize Sodium solution neutralization, concentration, water analysis, filtration, and dryin...
Embodiment 3 10
[0043] The synthesis of embodiment 3 testosterone undecanoate
[0044] 1) Under the protection of argon, put 10g of 4-androstenedione into 50ml of tetrahydrofuran, add 29.1ml of triethyl orthoformate, 1g of pyridinium hydrobromide, and keep warm at 50°C for reaction. After the reaction is completed, add 3ml of pyridine, Concentrate and filter to obtain 10.6 g of 3-ethoxy-androst-3,5-dien-17-one.
[0045] 2) Put 10.6g of 3-ethoxy-androst-3,5-dien-17-one into 106ml of tetrahydrofuran, add 10.6ml of triethylamine, 31.8g of sodium borohydride, keep warm at 20°C, and wait until the reaction is completed , water analysis, filtration, and drying to obtain 10.5g of 17β-hydroxy-3-ethoxy-androsta-3,5-diene
[0046] 3) Put 10.5g of 17β-hydroxy-3-ethoxy-androst-3,5-diene into 31.5ml of butanol, add 5.3ml of 20% dilute hydrochloric acid, keep warm at 20-30°C, after the reaction is completed, Neutralize with sodium hydroxide solution, concentrate, water analysis, filter, and dry to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com