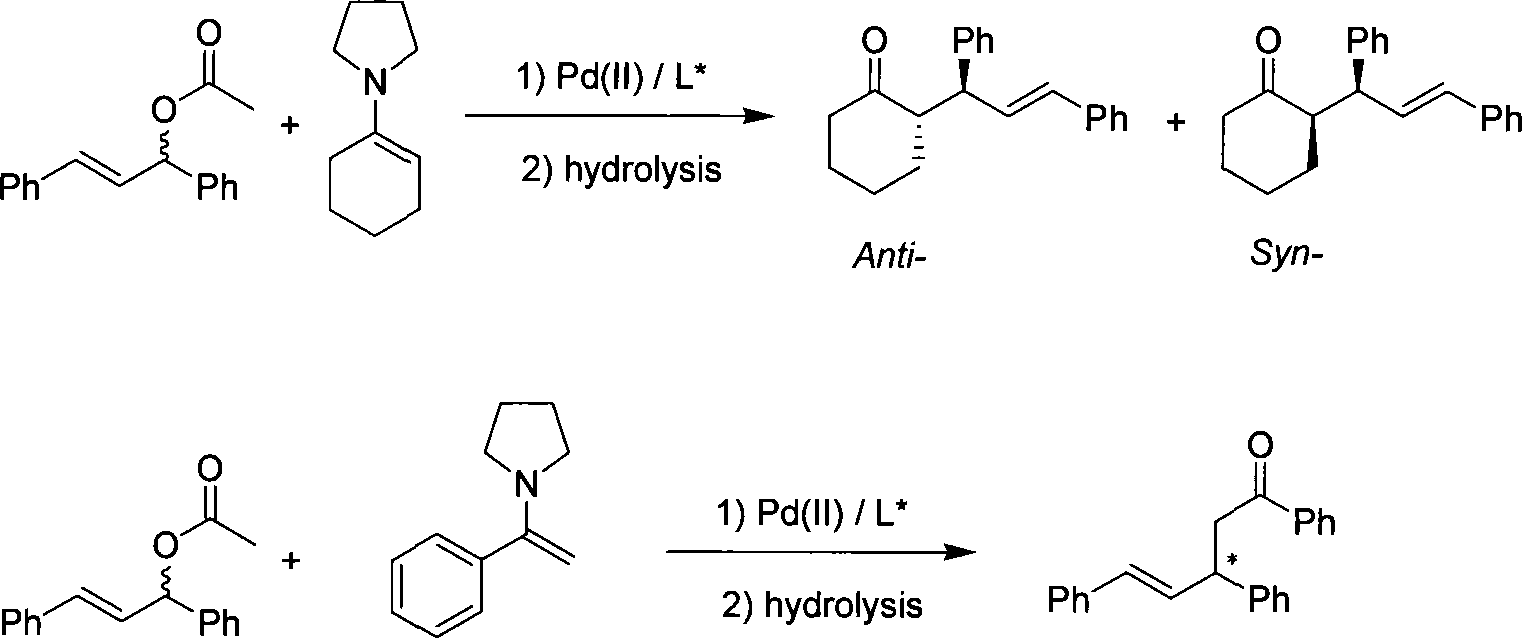
Method for application of enamine in palladium catalytic asymmetry allyl group alkylated reaction
An allyl alkylation and asymmetric technology, applied in asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., can solve the problems of expensive and dangerous reagents, harsh reaction conditions, troublesome operation, etc., and achieve high reaction Active, easy and safe operation, good application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Preparation of enamine
[0020] Cyclohexanone (20 g, 0.20 mol) was dissolved in cyclohexane (250 mL), then anhydrous magnesium sulfate (120 g) was added in one portion under nitrogen. The system was cooled down to 0° C., and pyrrolidine (84 mL, 1.0 mol, 5 equiv) was added thereto through a syringe for about 0.5 h. Continue to keep warm for 0.5h, and then stir the mixed system at room temperature overnight. Magnesium sulfate was removed by filtration and washed with ring-dried cyclohexane (3 x 50 mL). The combined filtrates were evaporated to remove the solvent under reduced pressure, and the residue was distilled under reduced pressure (69°C / 1mmHg) to obtain the product N-cyclohexenylpyrrolidine as a colorless liquid. Sealed under nitrogen and stored in refrigerator.
[0021] 1 H NMR (CDCl 3 , 400Hz): δ1.49-1.55(m, 2H), 1.62-1.68(m, 2H), 1.78-1.82(m, 4H), 2.05-2.09(m, 2H), 2.14-2.17(m, 2H) , 2.95-2.98(m, 4H), 4.24-4.26(m, 1H).
[0022] 2. Application in asymme...
Embodiment 2
[0025] 1. Preparation of enamine
[0026] Cyclohexanone (20 g, 0.20 mol) was dissolved in cyclohexane (250 mL), then anhydrous magnesium sulfate (120 g) was added in one portion under nitrogen. The system was cooled down to 0° C., and pyrrolidine (84 mL, 1.0 mol, 5 equiv) was added thereto through a syringe for about 0.5 h. Continue to keep warm for 0.5h, and then stir the mixed system at room temperature overnight. Magnesium sulfate was removed by filtration and washed with ring-dried cyclohexane (3 x 50 mL). The combined filtrates were evaporated to remove the solvent under reduced pressure, and the residue was distilled under reduced pressure (69°C / 1mmHg) to obtain the product N-cyclohexenylpyrrolidine as a colorless liquid. Sealed under nitrogen and stored in refrigerator.
[0027] 1 H NMR (CDCl 3 , 400Hz): δ1.49-1.55(m, 2H), 1.62-1.68(m, 2H), 1.78-1.82(m, 4H), 2.05-2.09(m, 2H), 2.14-2.17(m, 2H) , 2.95-2.98(m, 4H), 4.24-4.26(m, 1H).
[0028] 2. Application in asymme...
Embodiment 3
[0031] 1. Preparation of enamine
[0032] Cyclohexanone (20 g, 0.20 mol) was dissolved in cyclohexane (250 mL), then anhydrous magnesium sulfate (120 g) was added in one portion under nitrogen. The system was cooled down to 0° C., and pyrrolidine (84 mL, 1.0 mol, 5 equiv) was added thereto through a syringe for about 0.5 h. Continue to keep warm for 0.5h, and then stir the mixed system at room temperature overnight. Magnesium sulfate was removed by filtration and washed with ring-dried cyclohexane (3 x 50 mL). The combined filtrates were evaporated to remove the solvent under reduced pressure, and the residue was distilled under reduced pressure (69°C / 1mmHg) to obtain the product N-cyclohexenylpyrrolidine as a colorless liquid. Sealed under nitrogen and stored in refrigerator.
[0033] 1 H NMR (CDCl 3 , 400Hz): δ1.49-1.55(m, 2H), 1.62-1.68(m, 2H), 1.78-1.82(m, 4H), 2.05-2.09(m, 2H), 2.14-2.17(m, 2H) , 2.95-2.98(m, 4H), 4.24-4.26(m, 1H).
[0034] 2. Application in asymme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com