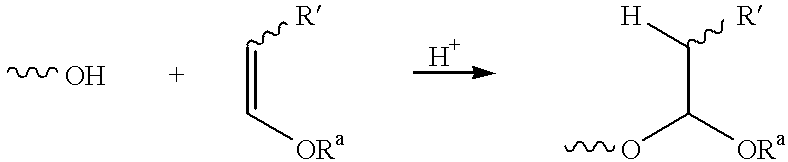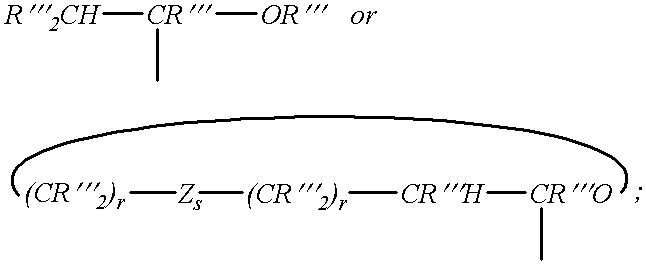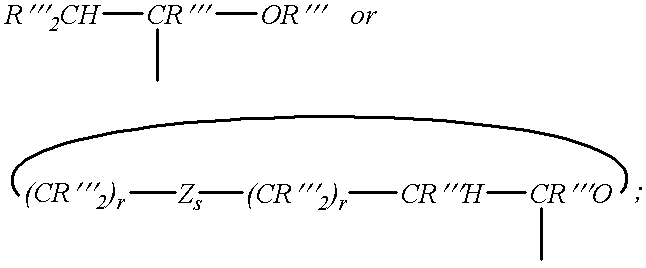Enol-ether capped polyethers and surfactants produced therefrom
a technology of enol-ether capped polyethers and surfactants, which is applied in the direction of group 4/14 element organic compounds, chemistry apparatus and processes, etc., can solve the problems of serious deficiency, acetate cap is not hydrolytically stable to acidic or alkaline water, and the williamson ether process has a number of problems, and achieves good potency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-- stability study
Example 1--Stability Study
To test the stability of a pyran cap, methyltriglycol (MTG) was capped with 2,3-Dihydro-4H-pyran using conditions described in U.S. Pat. No. 3,794,673 (Example 1). GC analysis indicates 97% capping was achieved. A sample of the resulting material (0.35g) was mixed with a conventional flexible foam formulation water / amine premix (1.15g of a 5.5:0.2 wt / wt water / A-200 amine mixture). Blends of acetoxy- and alkyl-capped MTG samples with conventional water / amine premix were similarly prepared (alkyl=C.sub.3 H.sub.5 and MTG-Acetate (MTG-Ac) which were prepared via conventional means). The hydrolysis at a room temperature of the different capping groups was followed by GC. The results in Table 1 clearly show the acetoxy group cleaving rapidly to about 15-20% uncapped which is approximately the level at which the liberated acetic acid would neutralize all of the amine in the mixture. The pyran-capped material exhibits excellent stability, comparable to the alkyl-ca...
example 2--
Capping Efficiency
Using a variety of acid catalysts, a broad spectrum of conventional polyethers of the type used for Copolymer synthesis were enol-ether capped. Excellent results were obtained even with random mixed feed polyethers such as 40 weight percent EO fluids that generally are approximately 95% 2.degree. alcohols (due to the sluggish nature of PO reaction during the last stages of polyether manufacture). Thus Table 3 shows that the technology readily caps even high molecular weight, high 2.degree. hydroxy polyethers very efficiently.
TABLE 3 Representative Capping Results. Acid % Polyether* Enol-Ether (% Excess) Catalyst Capping 100HA550 2,3-Dihydro-4H-pyran (50%) PTSA >98 Ethylvinyl Ether (14%) A-18 Resin >98 40HA1500 2,3-Dihydro-4H-pyran (50%) PTSA >98 Ethylvinyl Ether (14%) A-18 Resin -98 Ethylvinyl Ether (25%) H.sub.2 SO.sub.4 >98 40HA4000 2,3-Dihydro-4H-pyran (50%) PTSA >98 Ethylvinyl Ether (25%) H.sub.2 SO.sub.4 >98 75HA750 2,3-Dihydro-4H-pyran (50%) PTSA -98 *These a...
example 3--
Manufacture of Flexible Polyurethane Foam
In the examples that follow, all reactions involving the manipulation of organometallic compounds were performed in an inert atmosphere. Commercial reagents were used without additional purification.
The term potency refers to the ability of a surfactant to stabilize foam during its manufacture. High potency surfactants allow high heights of rise and only relatively small amounts of top collapse during foam manufacture. In general, higher rise and / or good rise at lower and lower use levels are desirable. The phrase "processing latitude" refers to the ability of a foam composition to tolerate changes in its ingredients or amounts thereof, while still producing product having the desired properties. In flexible foam applications, this is often reflected by relatively small changes in foam properties (such as breathability) at higher and higher surfactant or catalyst use levels. The terms breathability or airflow refer to the ability of a cured f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average molecular weight | aaaaa | aaaaa |
| average molecular weight | aaaaa | aaaaa |
| average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com