Fenitrothion hapten, artificial antigen, specified antibody and use thereof
A technology of fenitrothion and artificial antigen, which is applied in the field of immunology, can solve the problems that there are no research papers or works on the immune recognition mechanism of hapten molecules, and achieve the effect of good affinity and low cross-reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
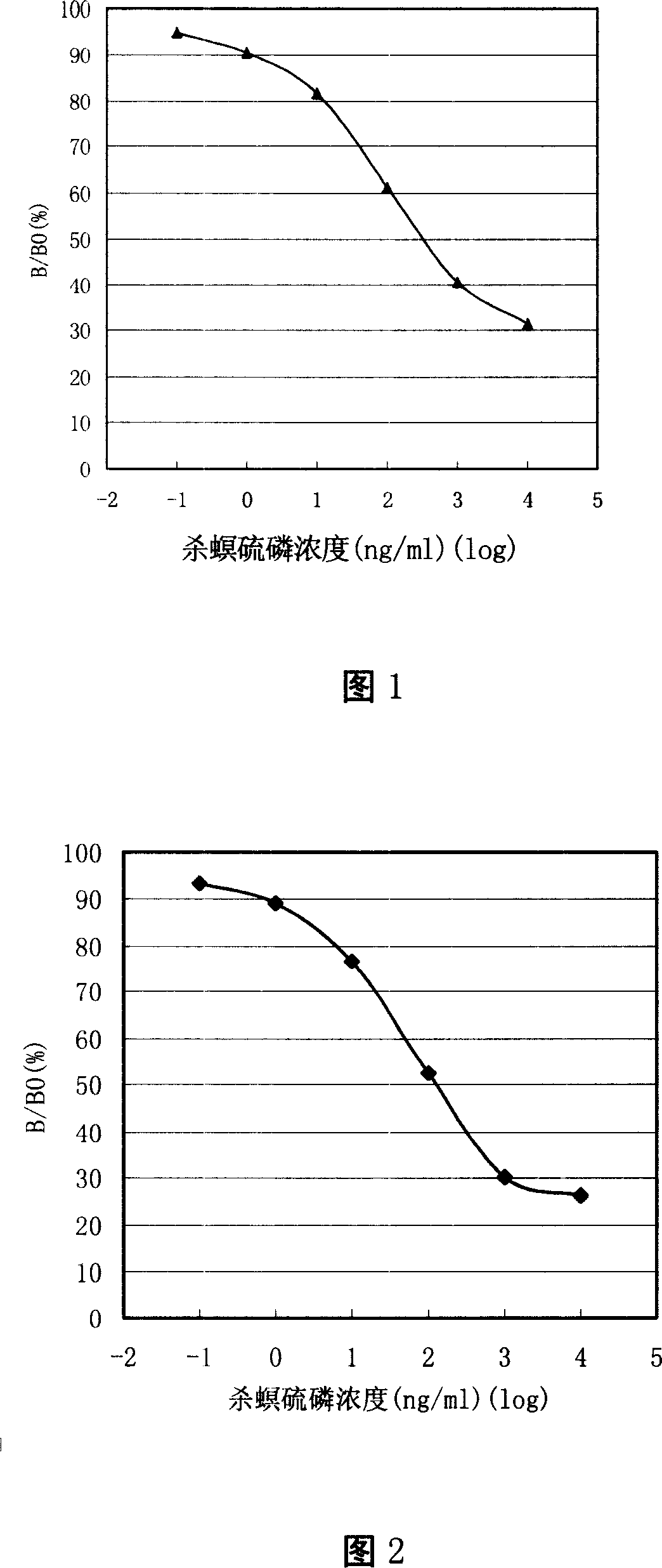
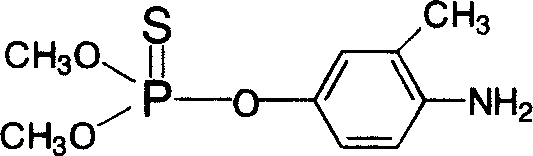
Image
Examples
Embodiment 1
[0023] Embodiment 1 Preparation of fenitrothion hapten, artificial antigen and antibody thereof
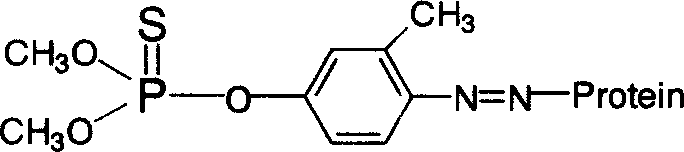
[0024] 1. Synthesis of hapten O, O-dimethyl-O-[3-methyl-4-aminophenyl] phosphorothioate (abbreviated as FNH2)
[0025] The synthesis of the hapten FNH2 is accomplished through the amination of fenitrothion. The synthetic route is as follows:
[0026]
[0027] The specific operation is as follows:
[0028] Take about 2.0 g of fenitrothion, dissolve it in 20 ml of ether, wash twice with 2×10 ml of cold 1% sodium carbonate solution, separate the ether layer, transfer it to a three-necked bottle, add acetic acid / hydrochloric acid (9:1 ) solution 20ml, add 4g zinc powder, reflux for 1.5h, and filter. Filter residue with 30mL CCl 4 Wash three times, then wash CCl with 30 ml of distilled water 4 phase, discarding the aqueous phase. Add 10% NaOH to the filtrate to adjust the pH value to neutral. At this time, a flocculent precipitate appears, and extract with ether 4×25ml. Dieth...
Embodiment 2
[0052] The assembly of embodiment 2 test kits
[0053] The present invention applies the prepared artificial antigen and antibody to the preparation of an immunoassay kit suitable for the detection of fenitrothion residues. In each well of the target plate, the coating antigen capable of specifically binding to the anti-fenitrothion antibody is coated with the coating solution, and blocked with 2% to 5% gelatin.
[0054] Reagents in the box include: washing solution (diluent), substrate diluent, anti-fenitrothion antibody (suitable for indirect ELISA), fenitrothion standard solution, horseradish peroxidase-labeled goat anti-rabbit antibody ( Suitable for indirect competition ELISA) or horseradish peroxidase-labeled anti-fenitrothion rabbit antibody (for direct competition ELISA), substrate, chromogenic substance and reaction termination solution, the preparation method is as follows:
[0055] Washing solution (reaction diluent) 40mL, the composition ratio is 0.1g of potassium...
Embodiment 3
[0062] Preparation of embodiment 3 enzyme-labeled antibody (improved sodium periodate method)
[0063] Weigh 5-10mg of horseradish peroxidase HRP and dissolve it in 1L of distilled water, add 0.2-0.4mL of newly prepared 0.1mol / L NaIO 4 The solution was stirred at room temperature in the dark for 15-30 minutes. Put the above solution into a dialysis bag, dialyze with 1 mmol / L acetate buffer solution, pH 4.4, overnight at 4°C. Use a carbonate buffer solution of pH 9.5 to raise the pH of the above-mentioned hydroformylated HRP solution to 9.0-9.5, then immediately add 1-2 mL of 0.01 mol / L carbonate buffer solution containing 10-20 mg of purified antibody, and keep away from room temperature. Stir lightly for 2 to 3 hours. Add 0.1-0.2mL of freshly prepared 4mg / mL NaBH4 solution, mix well, and place at 4°C for 2-3 hours. The reaction solution was put into a dialysis bag, dialyzed with 0.15 mol / L PBS, pH7.4, overnight at 4°C. Add an equal volume of saturated ammonium sulfate sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com