Preparation method and application of pendimethalin hapten and antigen
A pendimethalin and hapten technology, which is applied in the preparation methods of peptides, the preparation of organic compounds, the preparation of cyanide reactions, etc., to achieve the effects of high specificity, high sensitivity and rapid detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
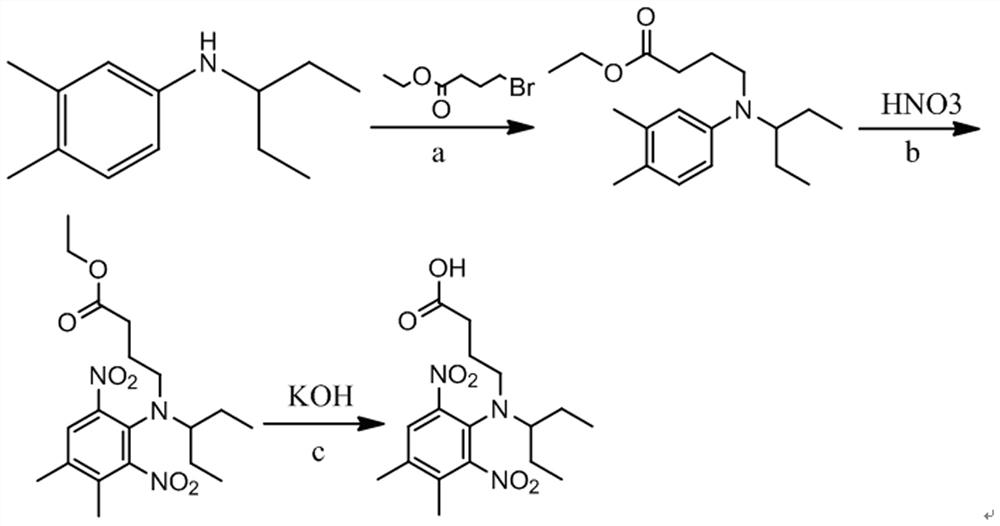
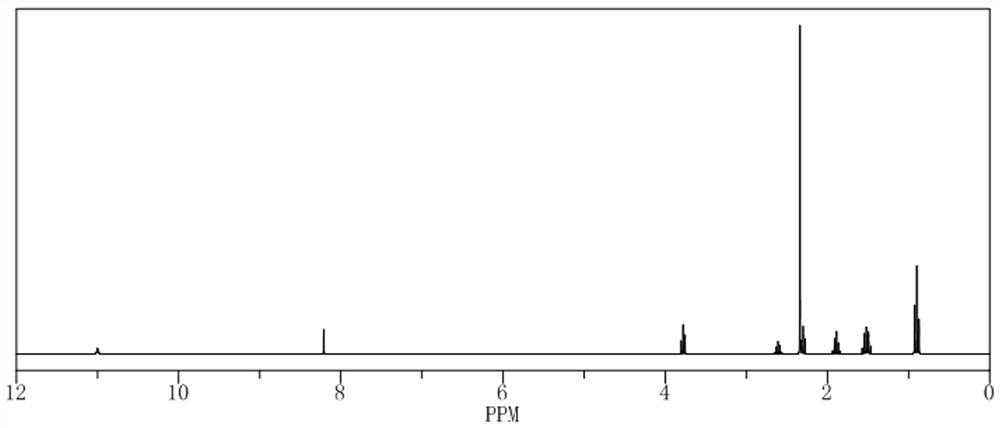
[0023] Example 1 Preparation of pendimethalin hapten
[0024] 1. Synthesis of pendimethalin hapten (see attached figure 1 )
[0025] 1) Take 1.00 g of N-(1-ethylpropyl)-3,4-dimethylaniline, add 80 mL of acetone to dissolve, add 0.35 g of potassium hydroxide, add 1.21 g of ethyl 4-bromobutyrate, and heat Reflux reaction for 3 h, TLC detection, all the raw materials were reacted, stop the reaction, cool to room temperature, rotary evaporate, remove acetone, add 60 mL water, add 50 mL dichloromethane for extraction, dry the organic phase with anhydrous sodium sulfate, evaporate to dryness and concentrate , put on a silica gel column, use petroleum ether / ethyl acetate with a volume ratio of 10:1 for elution and separation, and obtain 1.51 g of intermediate alkylated N-(1-ethylpropyl)-3,4-dimethylaniline, Yield 94.97%;
[0026] 2) Take 1.50 g of the intermediate alkylated N-(1-ethylpropyl)-3,4-dimethylaniline, add 5 mL of concentrated sulfuric acid to dissolve and clarify, slowl...
Embodiment 2
[0031] Example 2 Preparation of pendimethalin antigen
[0032] 1. Synthesis of pendimethalin immunogen
[0033] The pendimethalin hapten is conjugated with human serum albumin (HSA) to obtain the immunogen.
[0034] Take pendimethalin hapten 10 mg, add dimethyl sulfoxide (DMSO) to dissolve, add carbodiimide (EDC) 5.7 mg, stir, clarify, add N-hydroxysuccinimide (NHS) 3.8 mg, Stir and activate at room temperature for 3 h to obtain liquid A; take 50 mg of HAS and add 8 mL of 0.1 mol / L PB buffer solution to dissolve to obtain liquid B, slowly add liquid A to liquid B dropwise, and stir at room temperature for 5 h. Stop the reaction, dialyze with 0.02 M PBS buffer for 3 days, and change the medium three times a day to obtain the pendimethalin-HSA immunogen, which is aliquoted and stored at -20°C.
[0035] 2. Synthesis of Pendimethalin Coating Source
[0036] The pendimethalin hapten was coupled with ovalbumin (OVA) to obtain the coating source.
[0037] Take 6 mg of pendimethal...
Embodiment 3
[0040] Example 3 Preparation of Pendimethalin Monoclonal Antibody
[0041] 1. Obtaining hybridoma cells
[0042] 1) First immunization: fully emulsify the pendimethalin hapten-HSA conjugate (immunogen) with the same amount of complete Freund's adjuvant, and inject 6-week-old Balb / c mice subcutaneously, 0.2 mL each;
[0043] 2) Booster immunization twice: from the first immunization, booster immunization once every two weeks, with Freund's incomplete adjuvant instead of Freund's complete adjuvant, the method and dosage are the same as the first immunization;
[0044] 3) One week after the last booster immunization, the fundus vein blood was collected to measure the titer and inhibition. When there was inhibition and the titer reached more than 1:10000, the following last immunization was carried out: intraperitoneal injection of 0.1 mL of the immunogen solution without any adjuvant, and then executed three days later Mice, whose spleen was fused with myeloma cells;
[0045] 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com