Patents
Literature
107 results about "Enzyme-linked immunosorbant assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enzyme-linked immunosorbent assay (ELISA): ELISA stands for "enzyme-linked immunosorbent assay.". This is a rapid immunochemical test that involves an enzyme (a protein that catalyzes a biochemical reaction). It also involves an antibody or antigen (immunologic molecules).
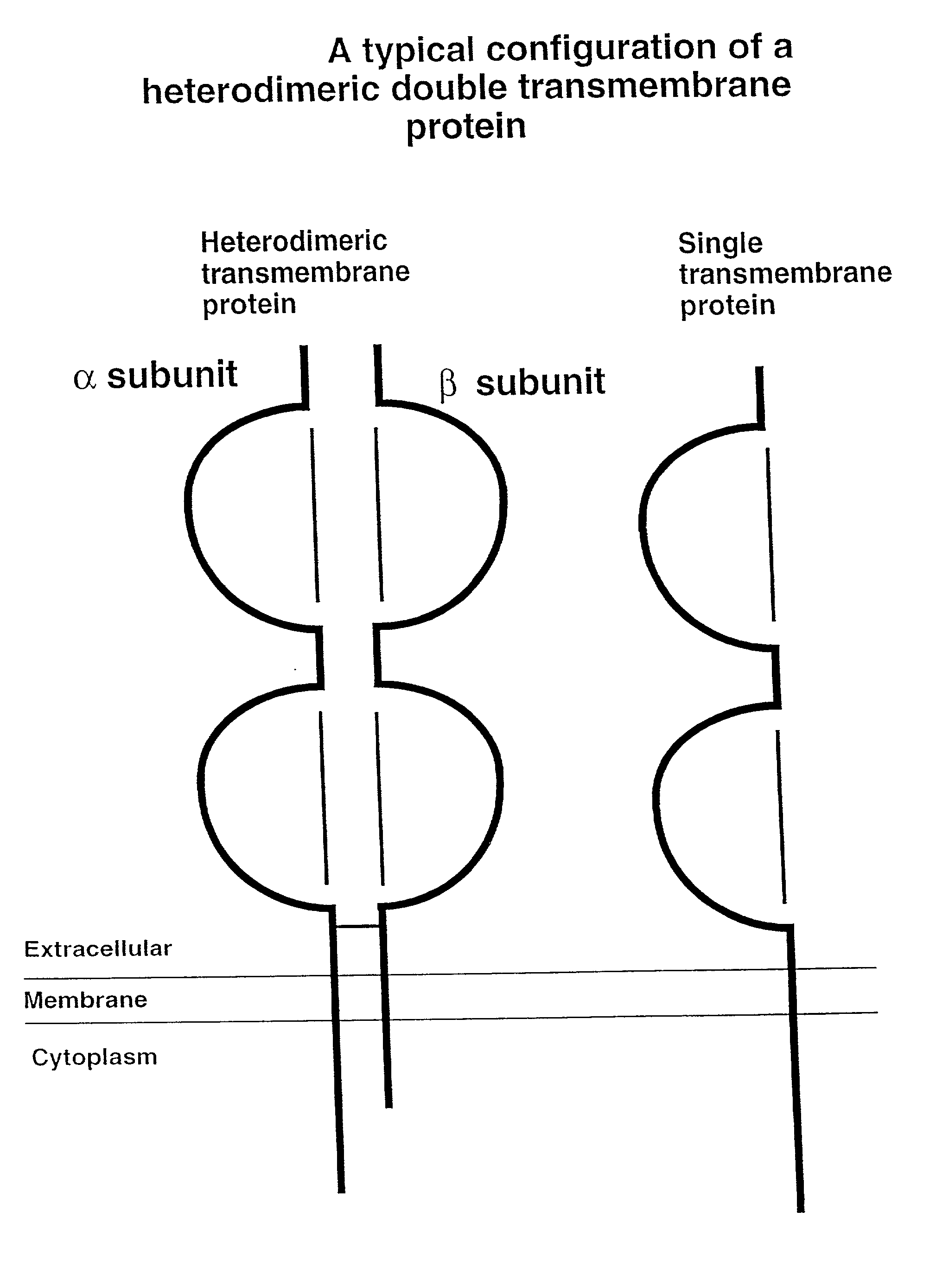
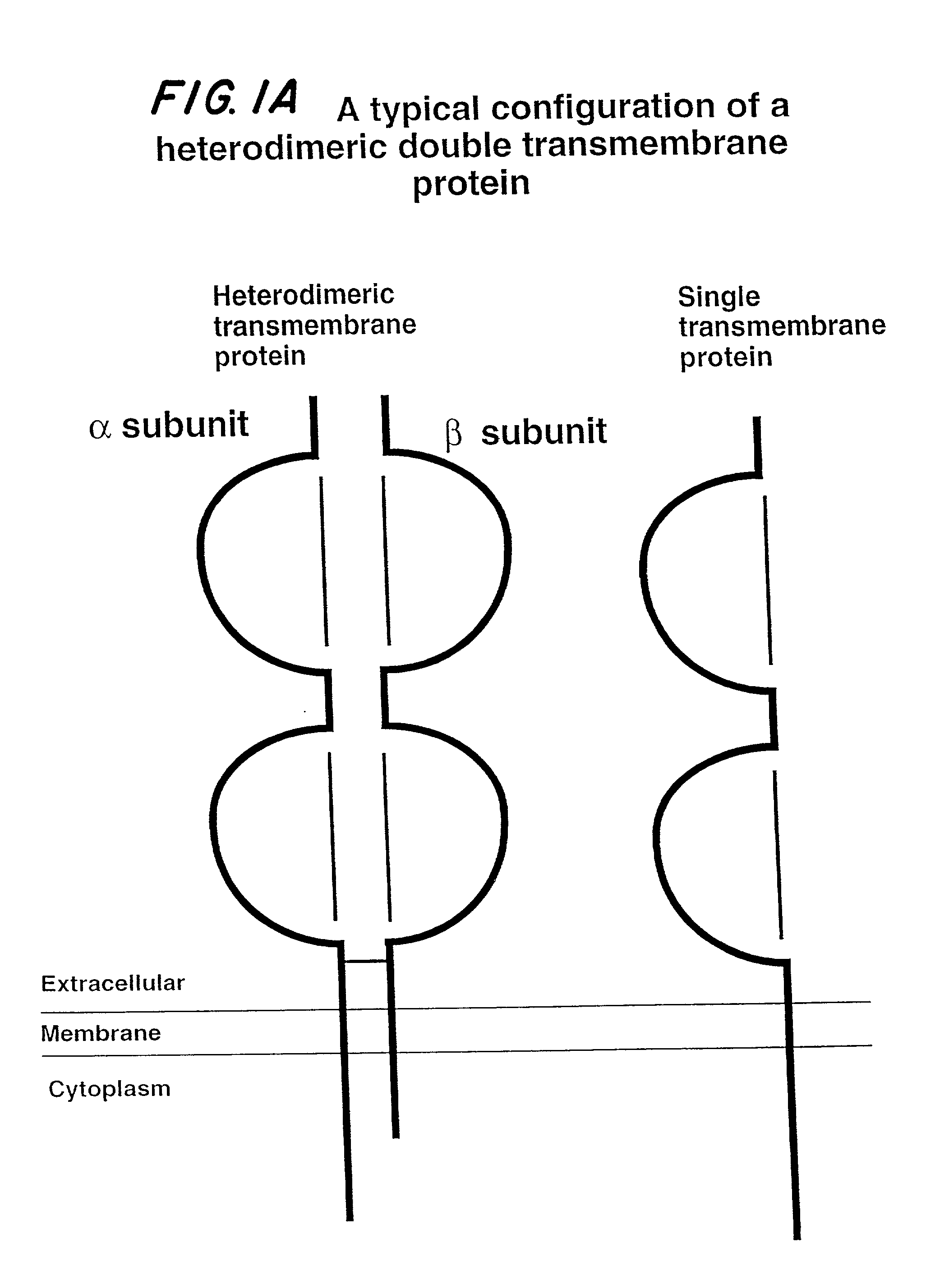
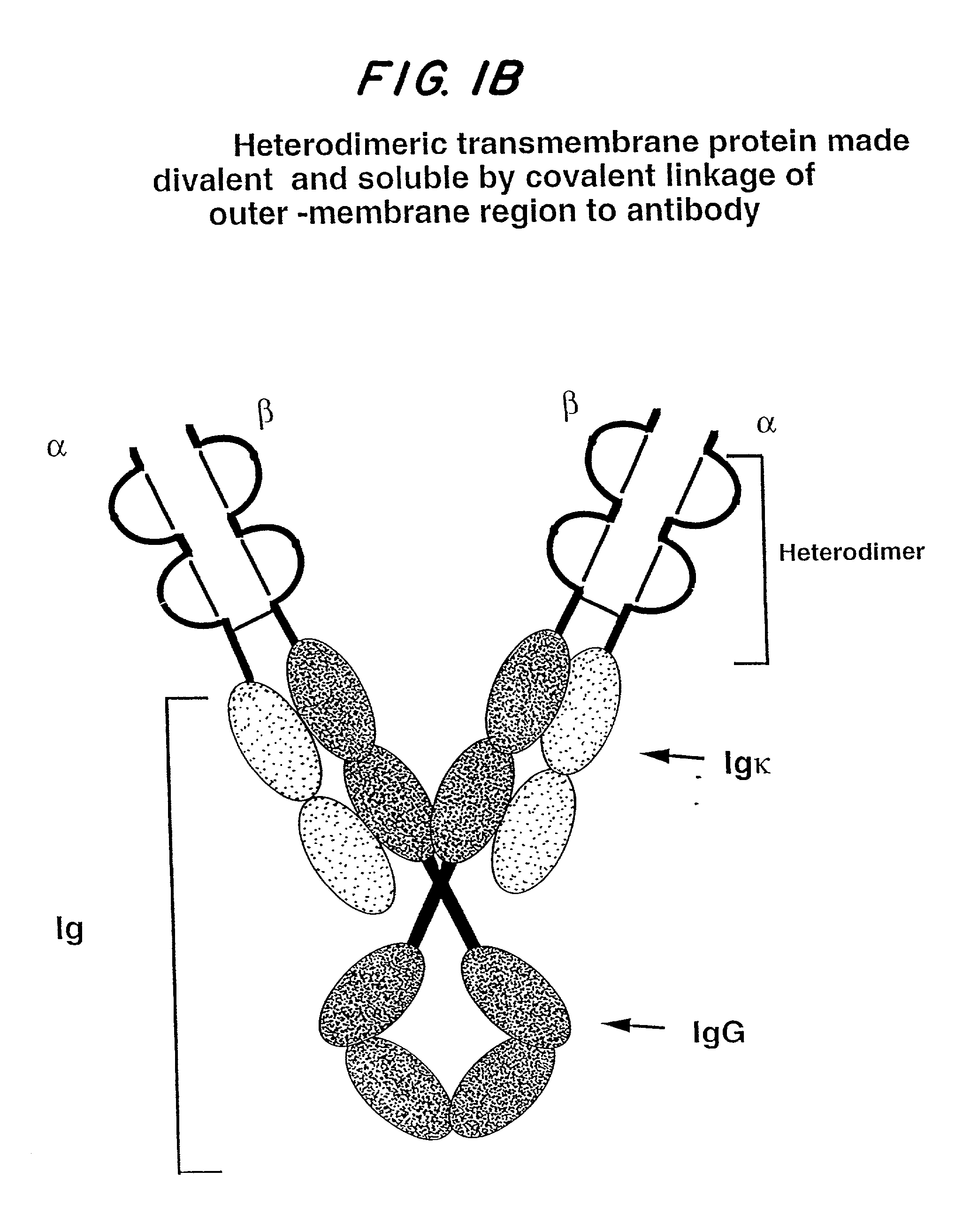
Soluble divalent and multivalent heterodimeric analogs of proteins
InactiveUS20020127231A1Well representedHigh affinityVirusesPeptide/protein ingredientsADAMTS ProteinsSpecific immunity
Specificity in immune responses is in part controlled by the selective interaction of T cell receptors with their cognate ligands, peptide / MHC molecules. The discriminating nature of this interaction makes these molecules, in soluble form, good candidates for selectively regulating immune responses. Attempts to exploit soluble analogs of these proteins has been hampered by the intrinsic low avidity of these molecules for their ligands. To increase the avidity of soluble analogs for their cognates to biologically relevant levels, divalent peptide / MHC complexes or T cell receptors (superdimers) were constructed. Using a recombinant DNA strategy, DNA encoding either the MHC class II / peptide or TCR heterodimers was ligated to DNA coding for murine Ig heavy and light chains. These constructs were subsequently expressed in a baculovirus expression system. Enzyme-linked immunosorbant assays (ELISA) specific for the Ig and polymorphic determinants of either the TCR or MHC fraction of the molecule indicated that infected insect cells secreted approximately 1 .mu.g / ml of soluble, conformnationally intact chimeric superdimers. SDS PAGE gel analysis of purified protein showed that expected molecular weight species. The results of flow cytometry demonstrated that the TCR and class II chimeras bound specifically with high avidity to cells bearing their cognate receptors. These superdimers will be useful for studying TCR / MHC interactions, lymphocyte tracking, identifying new antigens, and have possible uses as specific regulators of immune responses.
Owner:SCHNECK JONATHAN +1
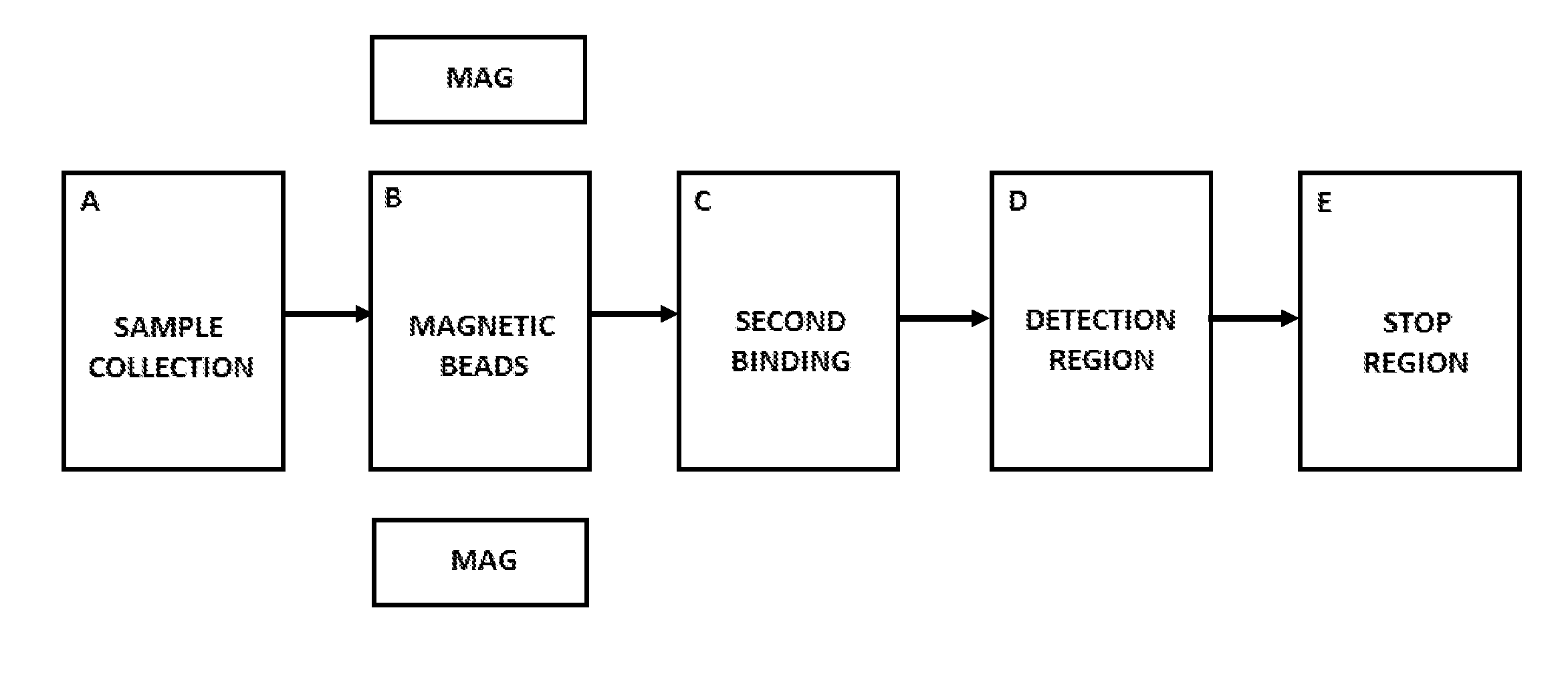
Discontinuous fluidic systems for point-of-care analyte measurement
ActiveUS20160195523A1Shorten speedEasy to separateMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPoint of careAir liquid interface
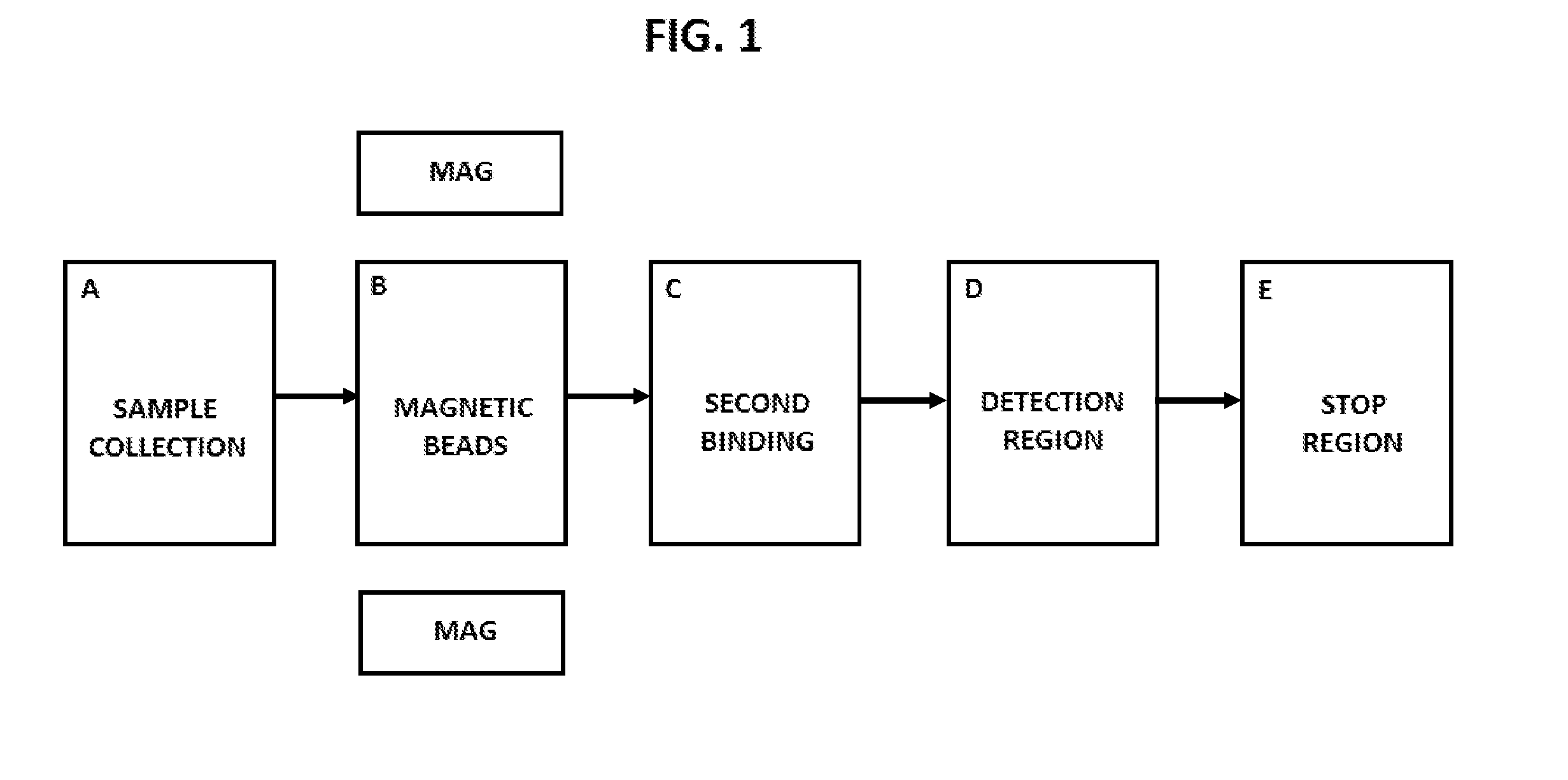
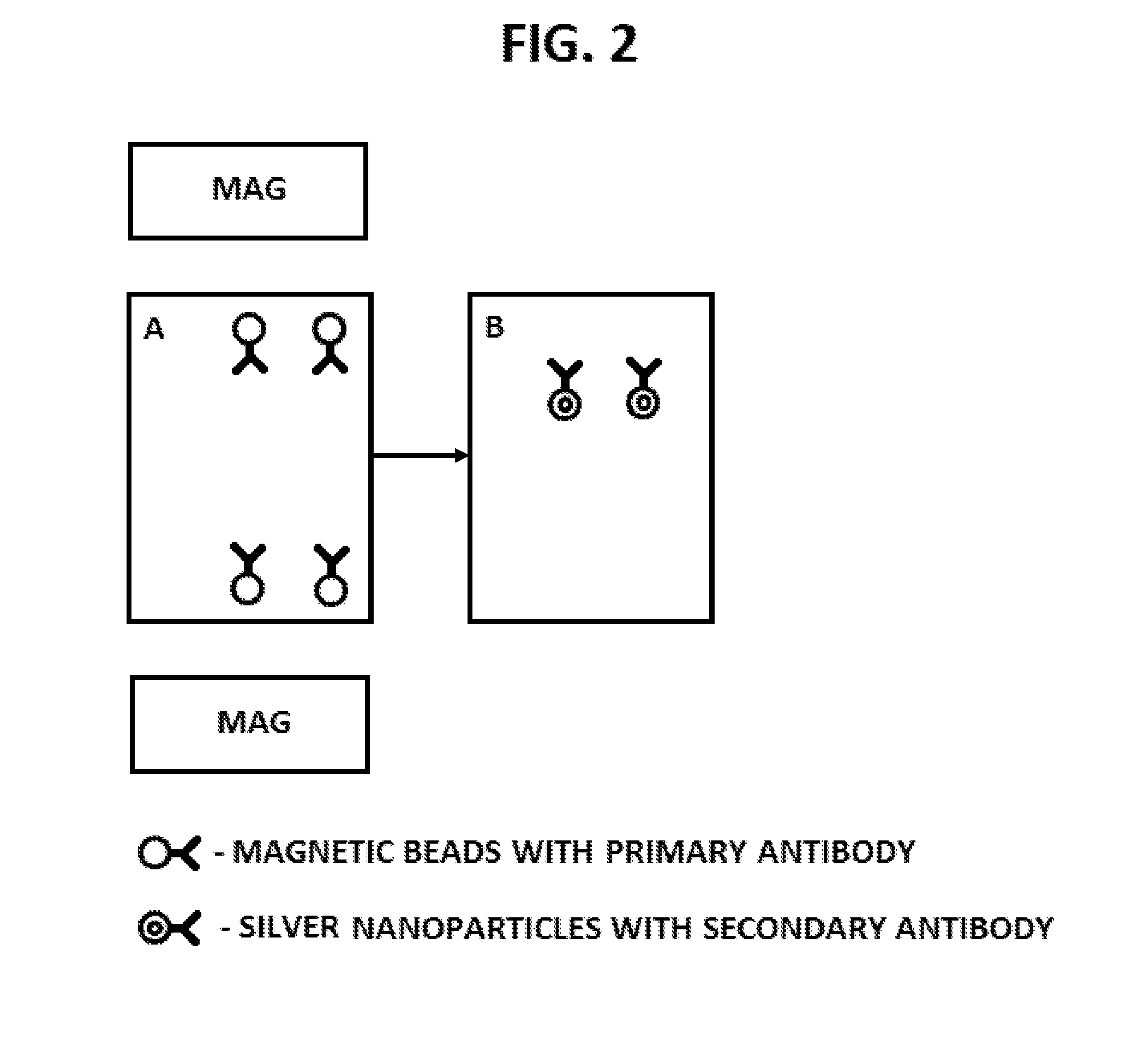
Disclosed herein are systems and methods for using the controlled movement of magnetic particles using controlled magnetic fields in a fluidic device containing separated fluidic regions to detect analytes in solution by immunoassay, such as an enzyme-linked immunosorbant assay (ELISA) for various medical and scientific applications. In order to achieve sequential exposure to the different chemical environments required in an immunoassay, magnetic particles are driven through fluid-containing chambers separated by air-gaps that may take the form of air bubbles or small open-air separations, for example. Externally controlled magnets coupled to actuators draw the flow of magnetic particles through air-liquid interfaces produced by microfluidic surface tension at the air-gap, washing the particles.
Owner:FANNIN INNOVATION STUDIO LLC +1
System for detecting infection in synovial fluid
ActiveUS20150011412A1Improve abilitiesBioreactor/fermenter combinationsPeptide librariesProteomics methodsRadioimmunoassay
The invention provides methods and systems for detecting a biomarker in a synovial fluid wherein the system also includes a control to ensure that the test sample is indeed synovial fluid. The biomarkers and the control for synovial fluid can be identified using proteomic methods, including but not limited to antibody based methods, such as an enzyme-linked immunosorbant assay (ELISA), a radioimmunoassay (RIA), or a lateral flow immunoassay.
Owner:CD DIAGNOSTICS
Method for the quantitative measurement of human acute phase serum amyloid A protein; recombinant protein; specific antibody
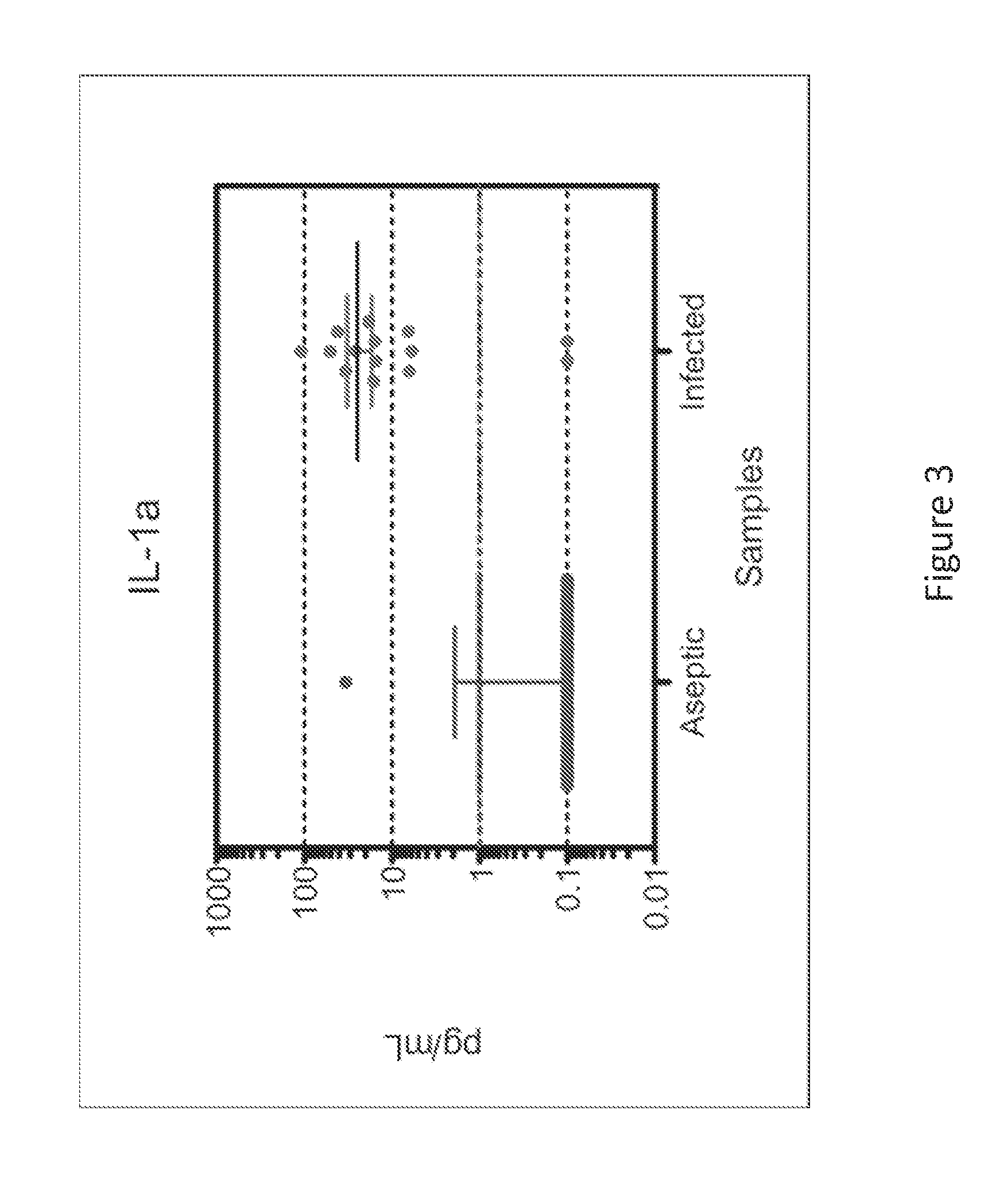
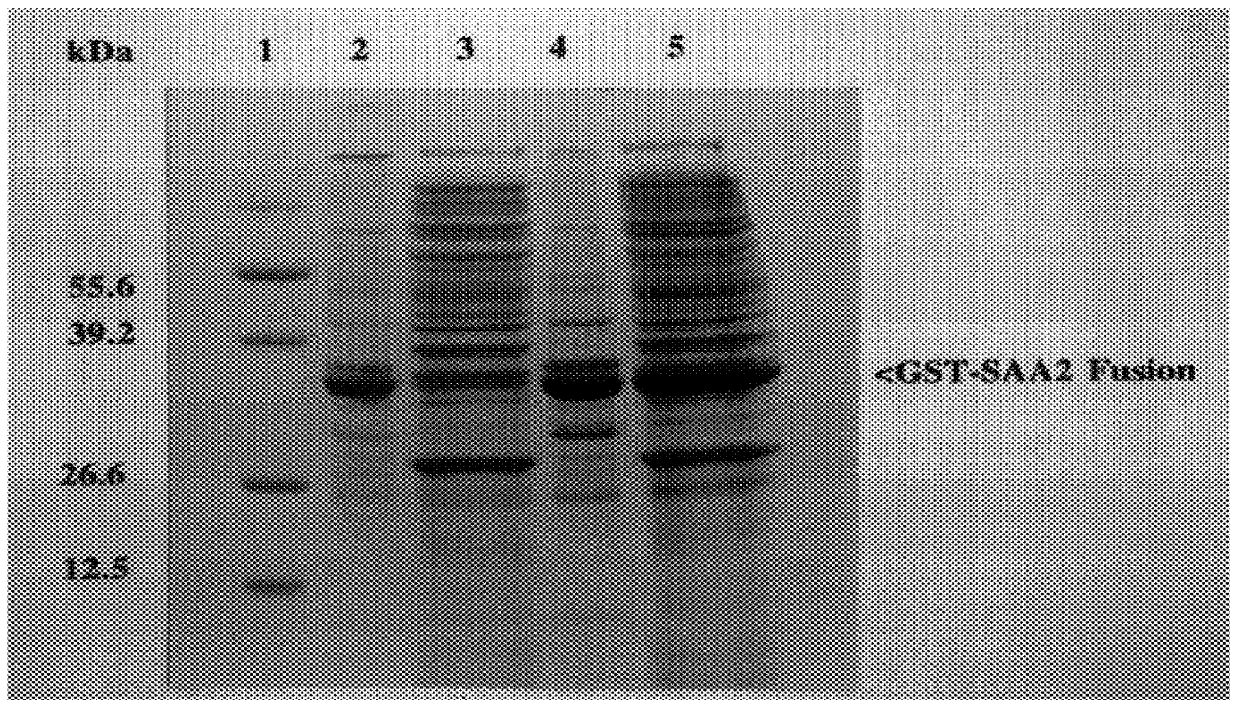
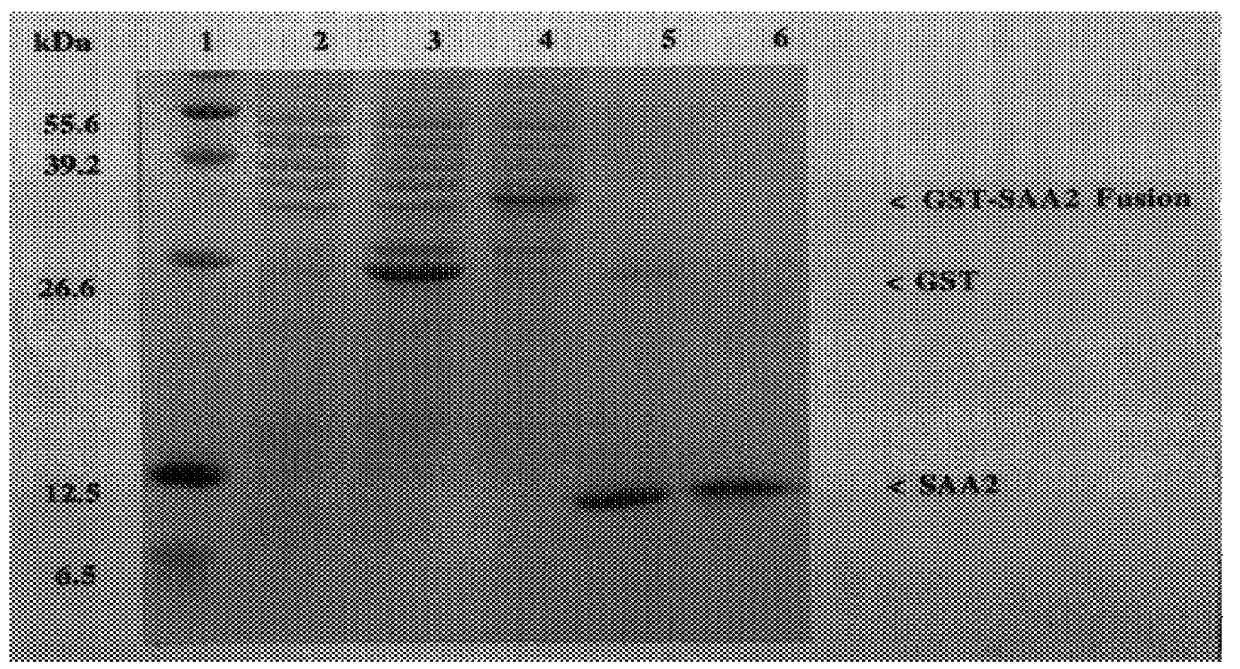
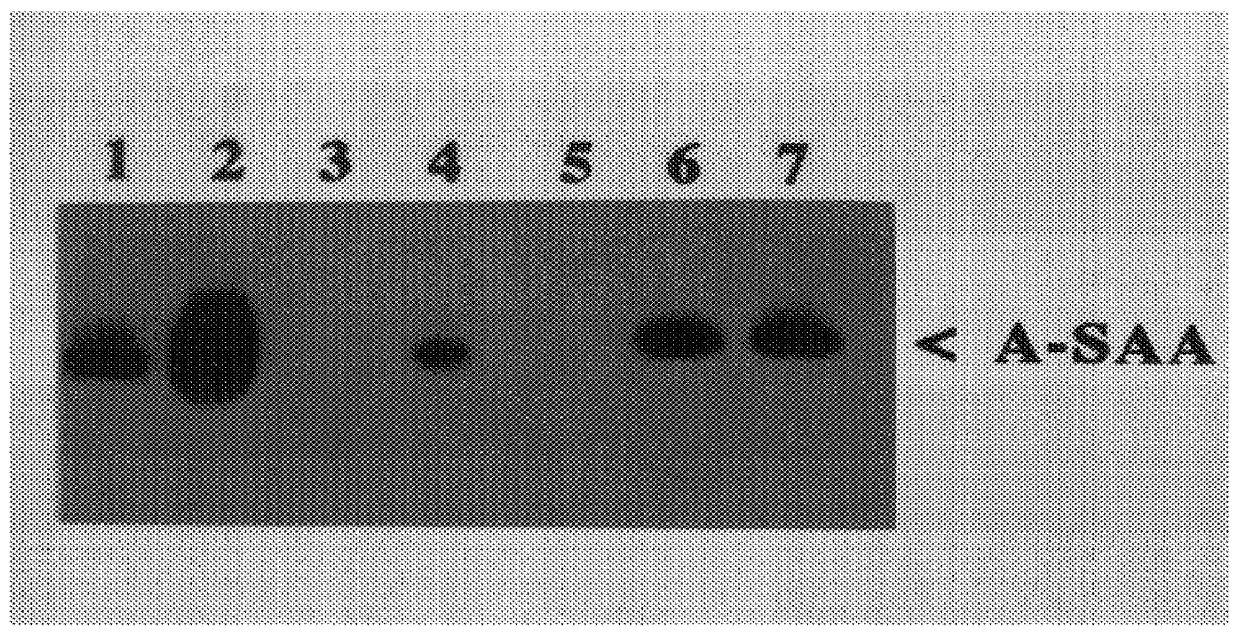
The present invention is drawn to a method for the quantitative determination of human acute phase serum amyloid A protein (A-SAA) comprising contacting a sample of a biological fluid with antibody specific for A-SAA, the sample being reacted with an organic solvent prior to or simultaneous with antibody contact. The organic solvent is suitably a C1 or C4 alcohol and the antibody can be used on the solid phase and as a component of the detection system of an enzyme linked immunosorbant assay. The method provides a sensitive and reliable measure of A-SAA and inflammatory status which can be used for the diagnosis and clinical management of both acute and chronic inflammatory conditions.
Owner:TRINITY COLLEGE DUBLIN
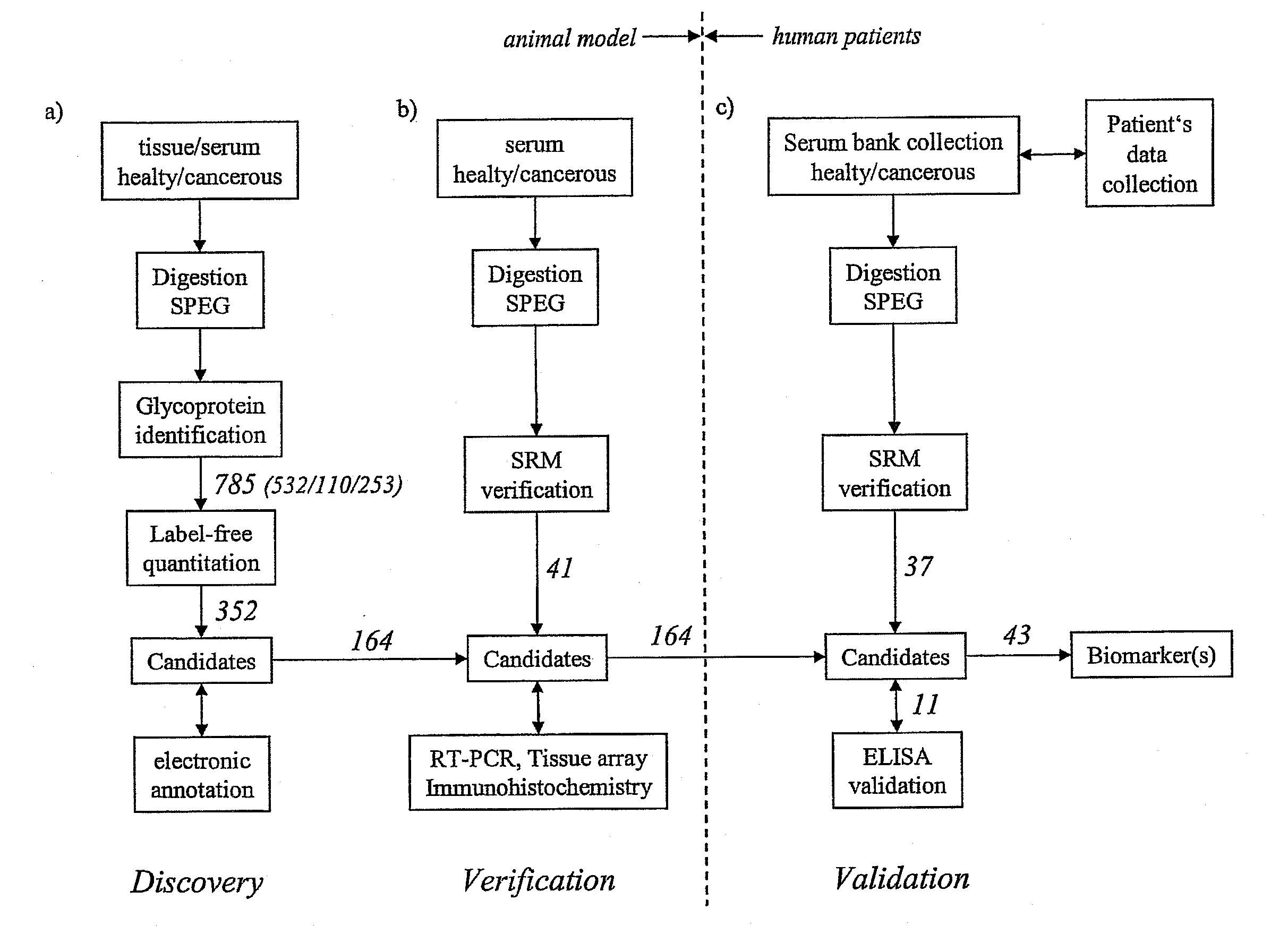
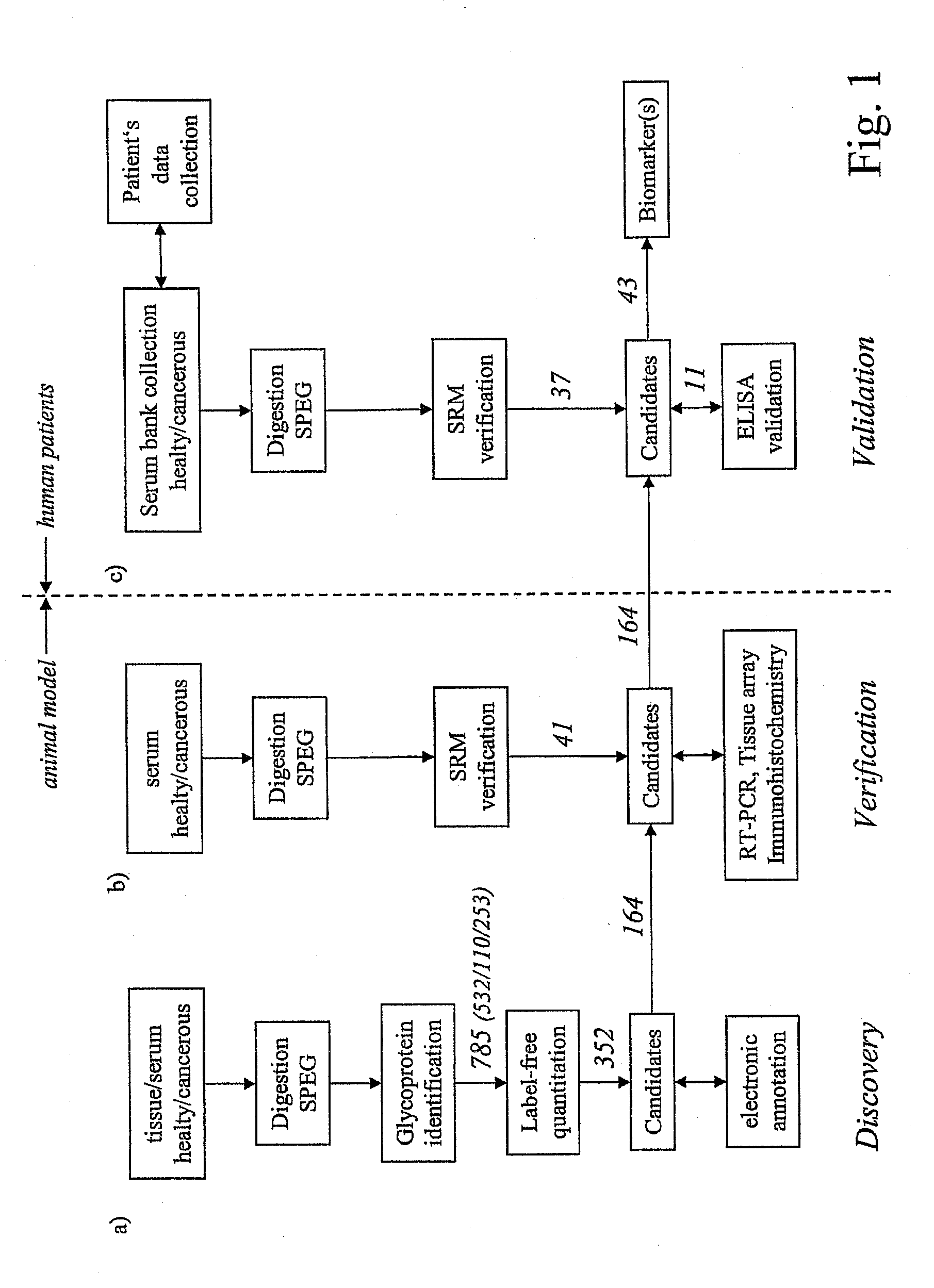
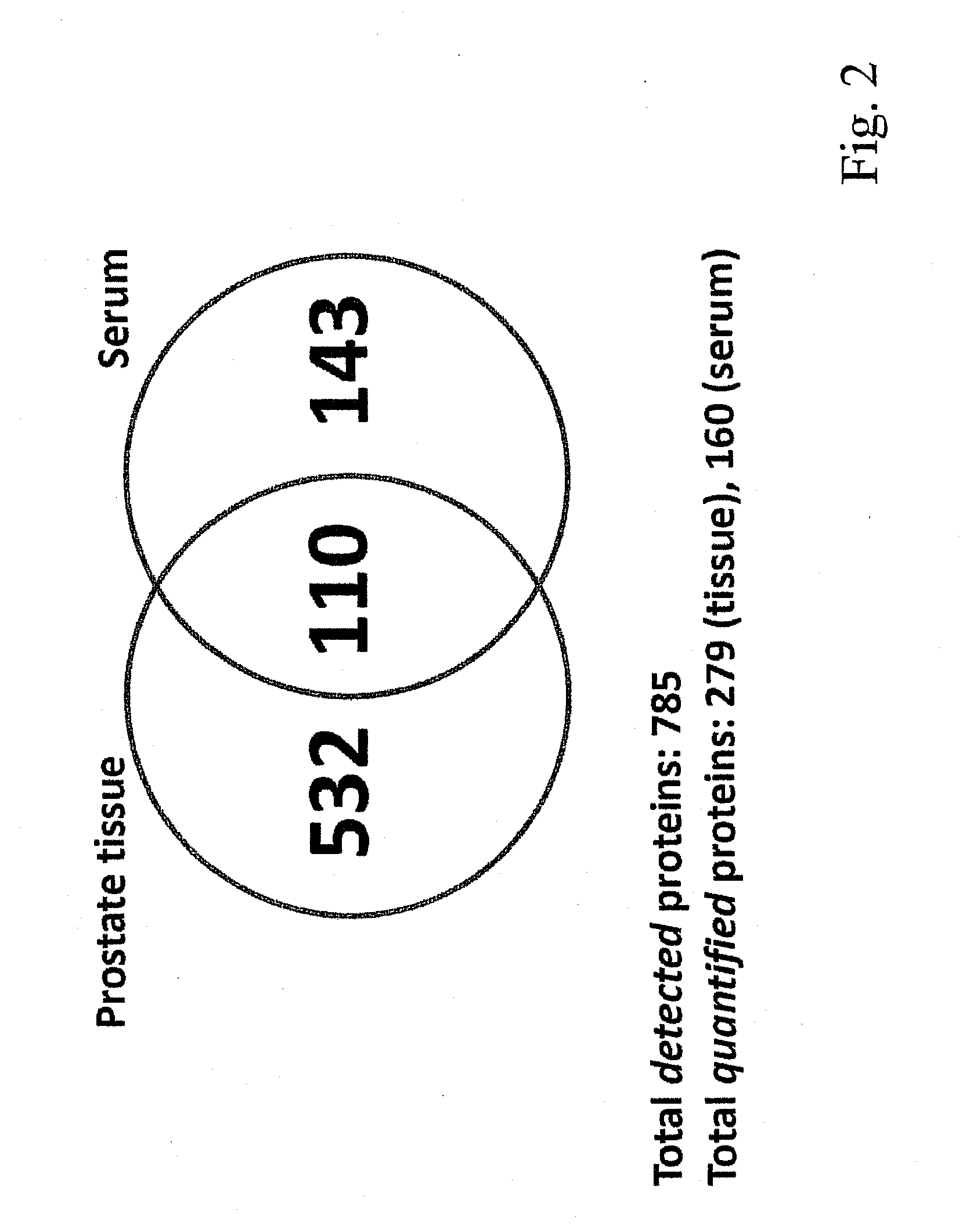
Method for biomarker and drug-target discovery for prostate cancer diagnosis and treatment as well as biomarker assays determined therewith
InactiveUS20110065605A1Improve accuracyImprove reliabilityMicrobiological testing/measurementLibrary screeningProtein proteinAntibody
The invention relates to a method for the determination of a cancer diagnostic / therapeutic biomarker assay and drug-targets including the following steps: (a) identification of potential candidate protein / peptide biomarkers and drug-targets based on the measurement of protein / peptide constituent concentrations in tissue sample proteomes as well as serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy non-human mammalian individuals as well as from cancerous non-human mammalian individuals and qualitatively selecting as potential candidate protein / peptide biomarkers those which show a pronounced differential behaviour between healthy and cancerous sample proteomes; (b) optional verification of the potential candidate protein / peptide biomarkers as identified in step (a) by quantitative mass spectrometric measurement of the potential candidate protein biomarkers in serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy non-human mammalian individuals as well as from cancerous non-human mammalian individuals and selecting as candidate protein / peptide biomarkers those which show a mass-spectrometrically measurable quantitative differential behaviour between healthy and cancerous sample proteomes; (c) validation of the candidate protein / peptide biomarkers as identified in step (a), or as optionally verified in step (b), by mass spectrometric measurement and / or antibody-based assays such as an Enzyme-Linked Immunosorbent Assay (ELISA) determination of the candidate protein biomarkers in serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy human individuals as well as from cancerous human individuals and selecting as protein / peptide biomarkers those which show a mass-spectrometrically measurable and / or antibody-based assay detectable differential behaviour between healthy and cancerous sample proteomes; (d) application of statistical methods to uncover single or groups of protein / peptide biomarkers as validated in step (c) as signatures for the detection of patients with cancer. The invention furthermore relates to specific biomarker assays for the highly reliable diagnosis of cancer, specifically of localized or non-localized prostate cancer, using human serum, plasma or any other derivatives of blood, or blood itself.
Owner:ETH ZZURICH +1
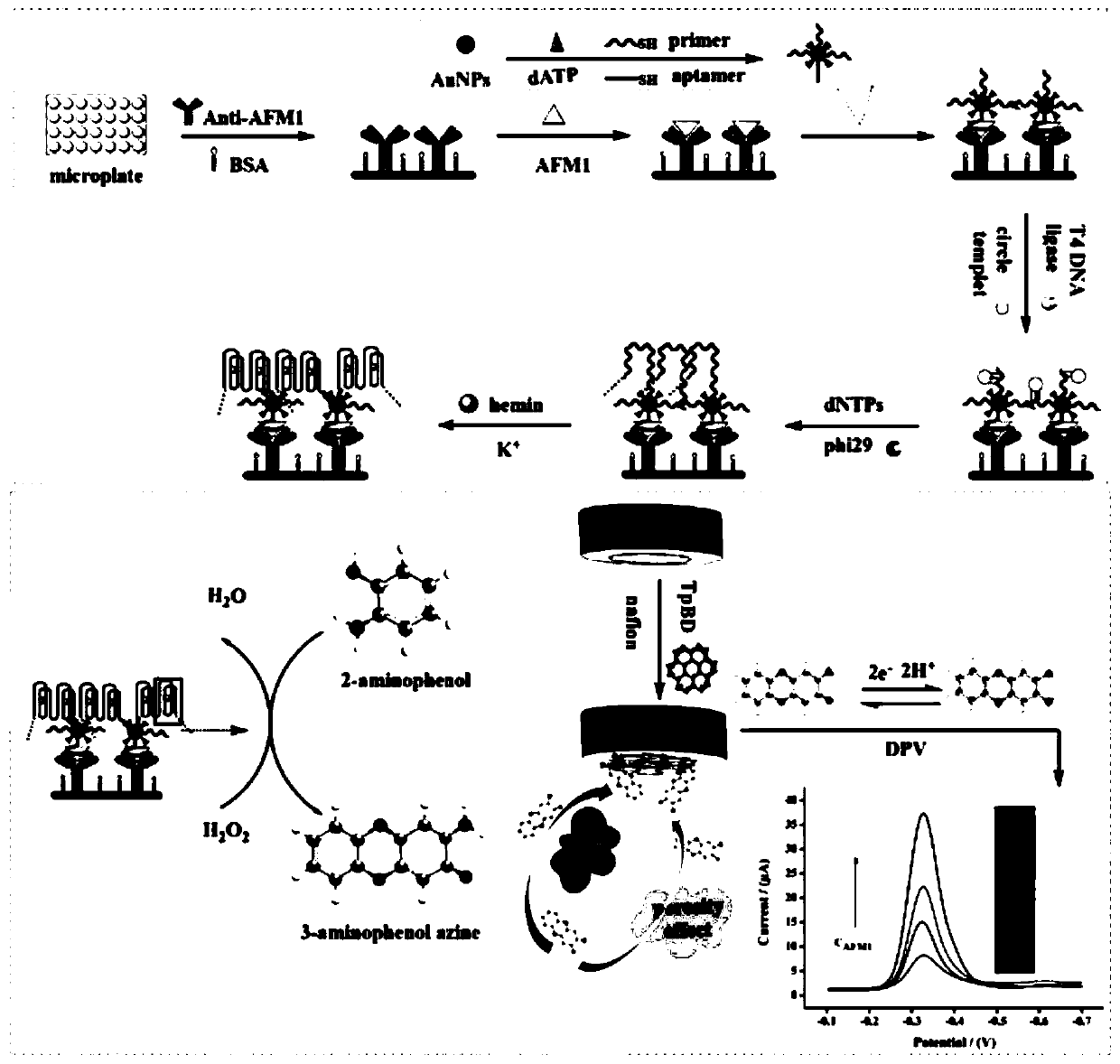
Rolling circle amplification DNA enzyme and covalent organic framework-based electrochemical-ELISA immunosensor
ActiveCN110736777AImprove throughputSensitive highMicrobiological testing/measurementMaterial analysis by electric/magnetic meansImmune profilingElisa method
The invention discloses a rolling circle amplification DNA enzyme and covalent organic framework-based electrochemical-ELISA immunosensor, and belongs to the technical field of electrochemical detection. An electrochemical method and enzyme-linked immunosorbent assay are combined, a nucleic acid signal is amplified on an ELISA plate, an electrode is modified with a functional nanometer material, linear dependence between an electrical signal of a reduction peak and aflatoxin M1 within a range being 0.5-80ng / mL is generated, an association coefficient is 0.993, and the detection limit is 0.15ng / mL (S / N=3). By the rolling circle amplification DNA enzyme and covalent organic framework-based electrochemical-ELISA immunosensor, the electrode passivation in traditional electrochemical analysis can be prevented, an ELISA signal also can be effectively amplified, amplification of a cascading signal is achieved, the detection limit is reduced, trace detection is achieved, and a high-sensitivity, high-selectivity and high-throughput electrochemical ELISA method is built.
Owner:JIANGNAN UNIV
Acetamiprid hapten and preparation method thereof as well as application
The invention discloses acetamiprid hapten and a preparation method thereof as well as application. The acetamiprid hapten is characterized in that the acetamiprid hapten is obtained by reacting N-noracetamiprid and aminobutyric acid; and the acetamiprid hapten is obtained by coupling acetamiprid hapten and carrier protein. The hapten prepared by the preparation method shows a specific acetamipridantigen determinant, so that a high-specificity acetamiprid monoclonal antibody is possibly screened out. The generated antibody is high in specificity and sensitivity, can be used for establishing an enzyme linked immunoadsorption assay method and a colloidal gold test paper quick measuring method, so that quick detection on acetamiprid in tobaccos and food is realized.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +2
Novel synthetic blocking reagents
ActiveUS20100317048A1Organic active ingredientsOrganic compound preparationPolyethylene glycolGlycol synthesis
The present invention concerns novel synthetic blocking reagents for the reduction of non-specific bindings in solid phase assays that rely on biological and specific recognition, e.g., in enzyme-linked immunosorbent assays (ELISAs). The invention provides the use of compounds as blocking reagents as well as kits comprising these compounds. The compounds comprise one or more poly(ethylene glycol) moieties, one or more alkyl- or aminoalkyl groups and another unit bridging the aforementioned groups, wherein the compound comprises at least one amino group.
Owner:FORSCHUNGSZENTRUM BORSTEL
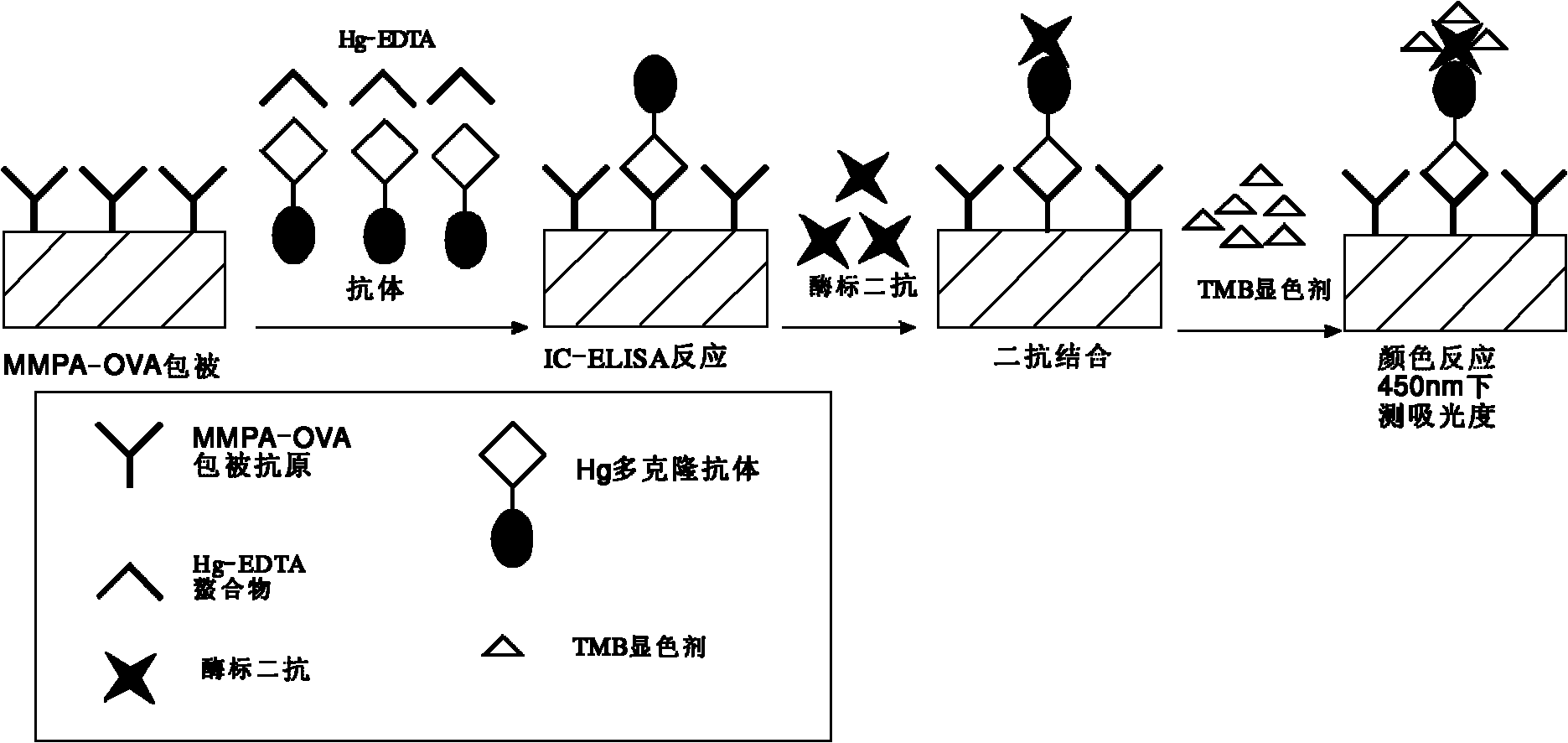
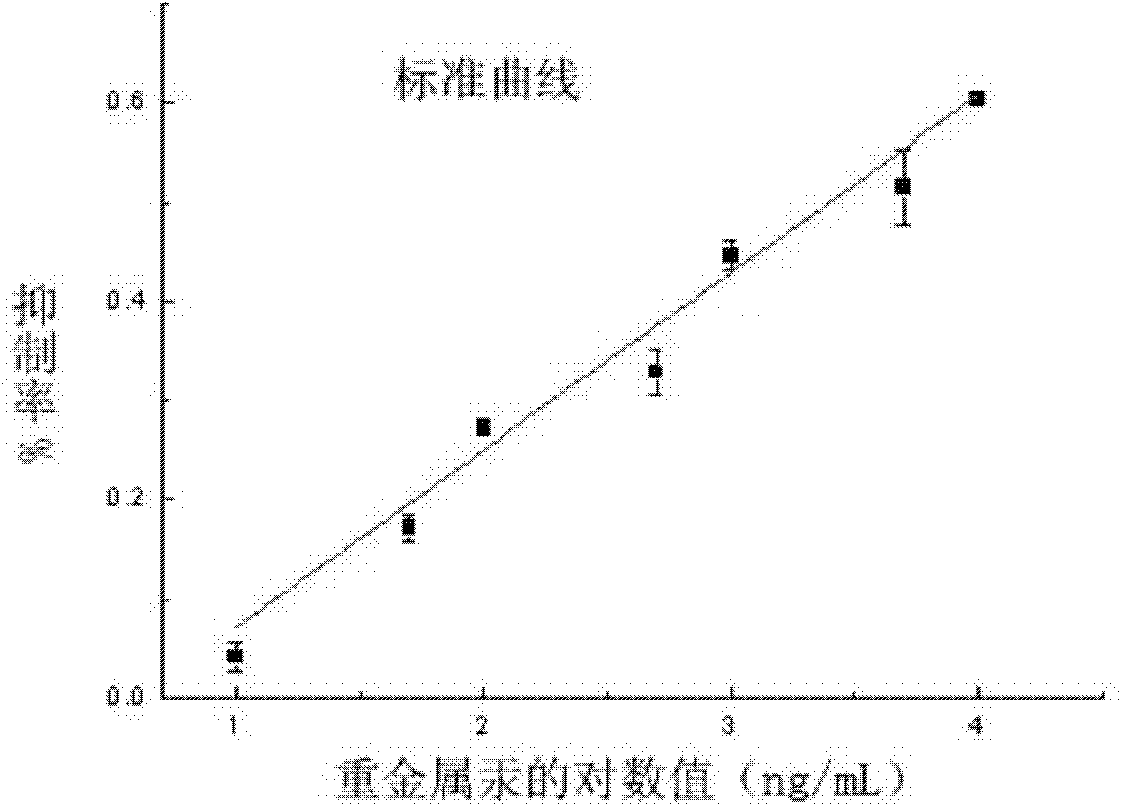
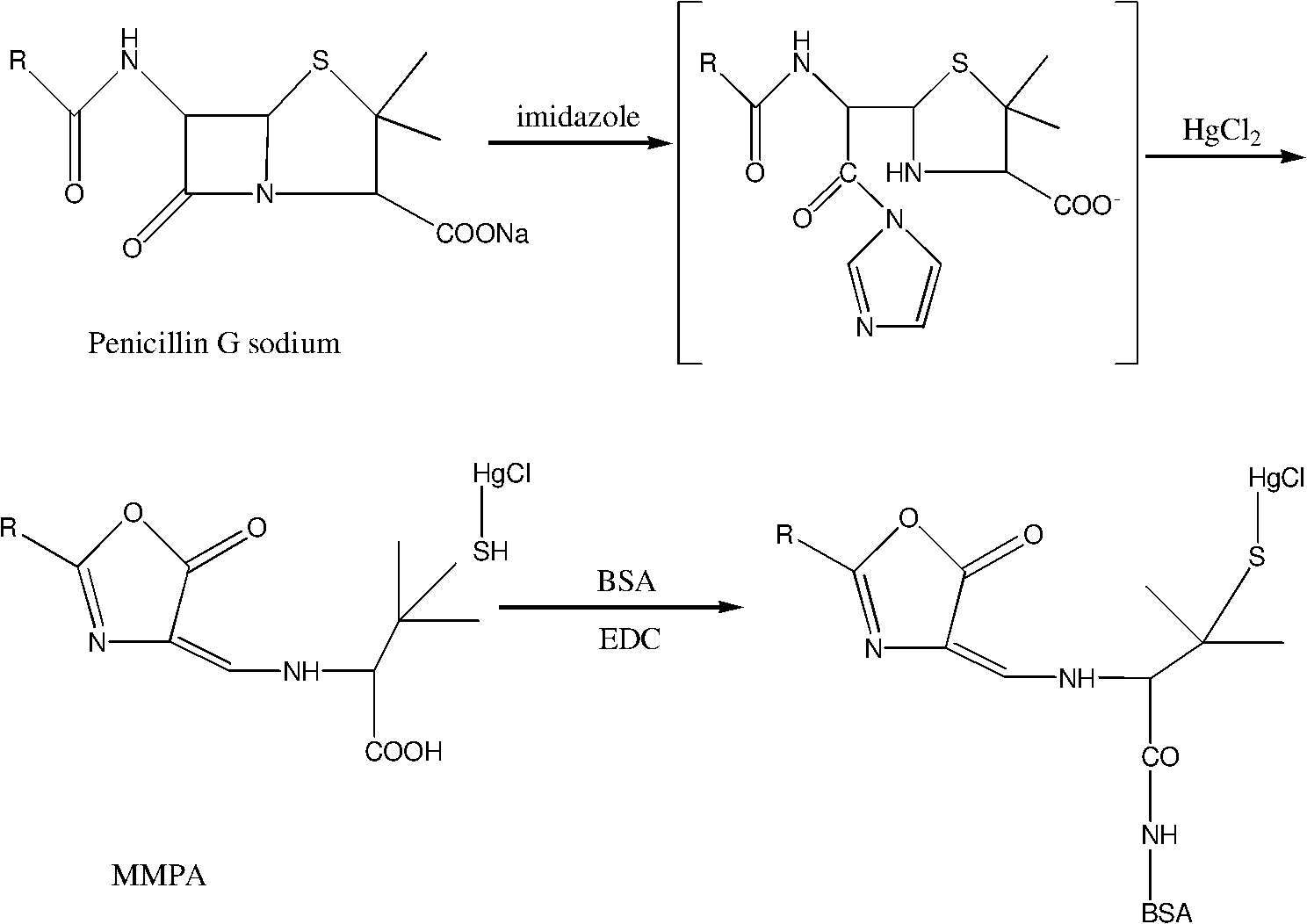
Preparation and enzyme-linked immunosorbent assay method for heavy metal mercury polyclonal antibody
ActiveCN101885775AHigh specificityQuick checkImmunoglobulinsBiological testingPenicillin G SodiumIon
The invention discloses preparation and an enzyme-linked immunosorbent assay method for heavy metal mercury polyclonal antibody in the technical field of heavy metal detection. The polyclonal antibody is prepared for the enzyme-linked immunosorbent assay by the following steps of: bonding the heavy metal mercury at one end of penicillin G sodium serving as a dual-functional chelating agent; coupling the other end with high molecular carrier proteins, namely bovine serum albumin and ovalbumin, to form immunogen MMPA-BSA and envelope antigen MMPA-OVA of mercury-chelating agent-protein; and finally emulsifying and immunizing the immunogen MMPA-BSA to prepare the polyclonal antibody. The prepared antibody has high specificity for the mercury ions, a cross reaction ratio with all other metals of less than 0.001 percent apart from the cross reaction ratio with cadmium of 7.9 percent and an adding recovery ratio of between 91.4 and 120 percent; and thus the antibody can be used for the assayof the heavy metal mercury in a water sample and can also be developed into rapid immunological detection technology for rapidly detecting the heavy metal mercury in other samples such as agricultural products and the like by combining certain pre-processing technology.
Owner:SHANGHAI JIAO TONG UNIV
Flow system and methods for digital counting
ActiveUS20180067113A1Reduce evaporationEasy to separateLaboratory glasswaresBiological testingAnalyteMolecular analysis
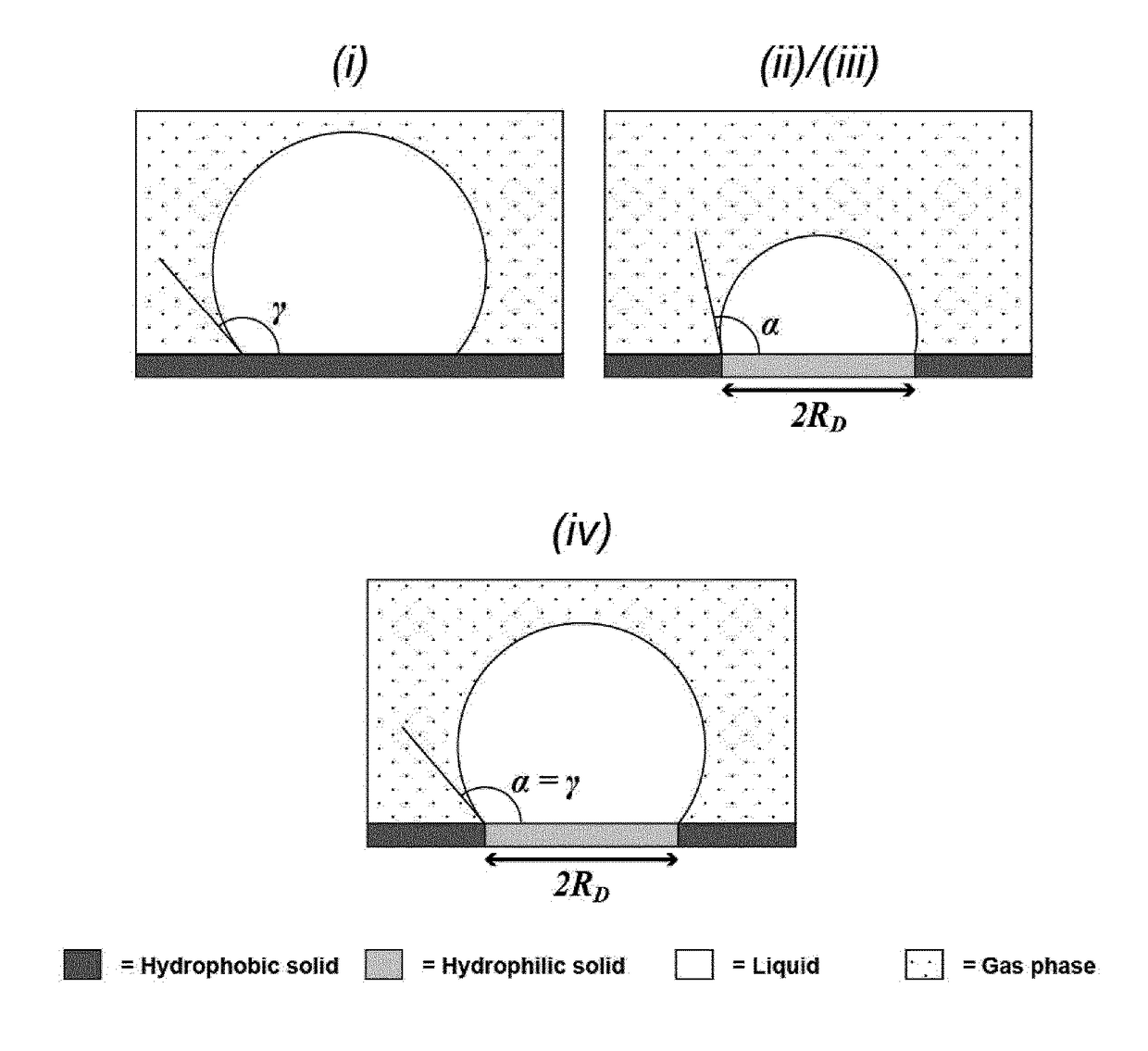
The present invention relates to methods and systems for testing for the presence of a material such as one or more analyte types within a sample and more particularly, for improved single enzyme-linked immunosorbent assay (sELISA) testing as well as other variants of single-enzyme linked molecular analysis (SELMA). The invention involves flow systems for digital counting of analytes with at least one opening (inlet / outlet). A support with hydrophilic and hydrophobic patches preferably harbours capture probes immobilised on the hydrophilic features. Nano-to-attoliter droplets are formed on the hydrophilic features. A gas phase (called gas phase seal) is applied to prevent / reduce evaporation from the droplets.
Owner:SELMA DIAGNOSTICS APS
Preparation methods and application of pendimethalin hapten and antigen
ActiveCN109232286AQuick checkRetain chemical structureOrganic compound preparationOvalbuminDimethylaniline N-oxidePotassium hydroxide
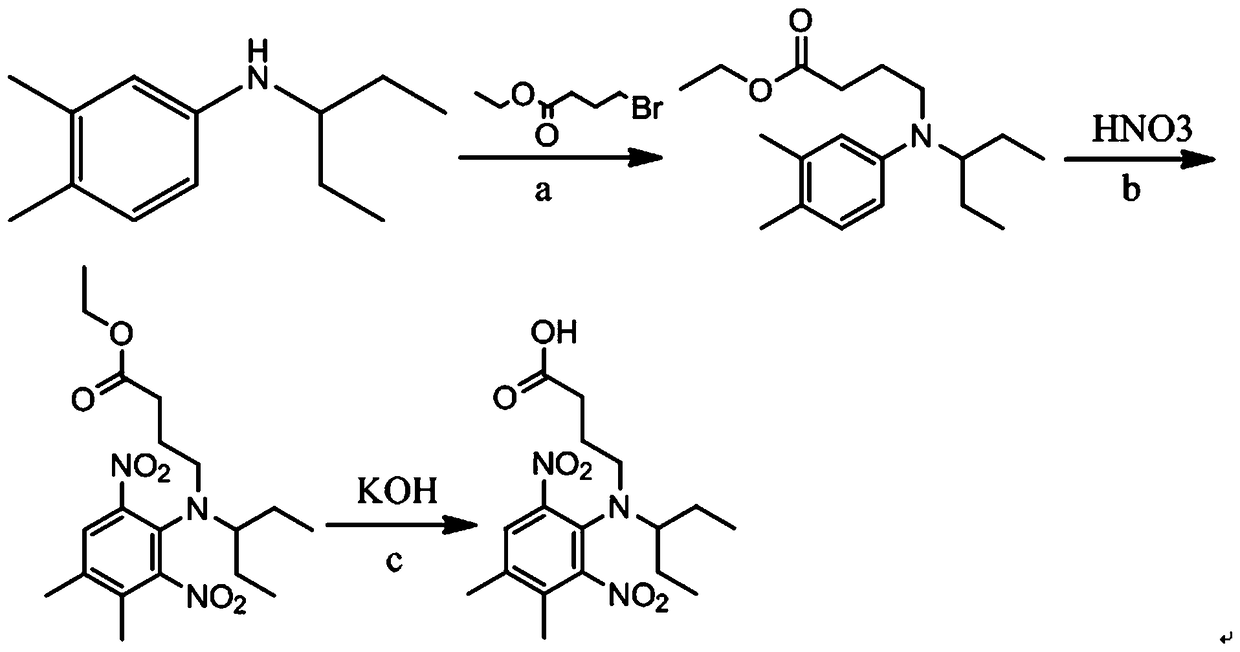
The invention provides preparation methods and application of pendimethalin hapten and antigen. The pendimethalin hapten is characterized by being prepared by taking N-(1-ethylpropyl)-3,4-dimethylaniline and 4-ethyl bromobutyrate to react to generate alkylated N-(1-ethylpropyl)-3,4-dimethylaniline, taking the alkylated N-(1-ethylpropyl)-3,4-dimethylaniline and concentrated nitric acid to react togenerate nitro-alkylated N-(1-ethylpropyl)-3,4-dimethylaniline, and then reacting with potassium hydroxide; the pendimethalin antigen is prepared by coupling the pendimethalin hapten and carrier protein. The antigen prepared by the invention has a specific pendimethalin antigen determinant, so that a high-specificity pendimethalin monoclonal antibody is possibly screened. The produced antibody hashigh specificity and high sensitivity and can be used for establishing an enzyme-linked immunosorbent assay method and a colloidal gold test strip determination method, so that rapid detection of pendimethalin in tobaccos and foods is realized.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +2
Differential immunoassay for prrs vaccine antibody
The present invention relates to immunoassays for serologically differentiating animals naturally infected with PRRS virus from animals vaccinated against PRRS. The immunoassays provide detection of at least a portion of the N terminal region of the 2b portion of PRRSV. The immunoassay is preferably an enzyme-linked immunosorbent assay (ELISA).
Owner:IOWA STATE UNIV RES FOUND
Utilization of nucleotide probes for the measurement of specific mRNA for the molecular diagnosis of autosomal recessive spinal muscular atrophy
InactiveUS20030049627A1Sugar derivativesMicrobiological testing/measurementSingle-strand conformation polymorphismNucleotide
The present invention concerns the development of a quantitative method for the molecular diagnosis of autosomal recessive spinal muscular atrophy (SMA) by measuring the amount of cytosolic mRNA from human muscle cells. Both the procedure using radioactive material and the Enzyme-Linked Immunosorbent Assay (ELISA) nonradioactive method were developed using 32P-dCTP labeled and biotinylated nucleotide probes. The results obtained demonstrate that the measurement of mRNA could be used as a quantitative method for the molecular diagnosis of SMA. There was a perfect concordance of the results obtained between the procedure using radioactive material, the ELISA method and the single strand conformation polymorphism (SSCP) analysis regarding the negative and positive SMA samples. The methods developed in this study may be applicable to the diagnosis (detection of homozygous and heterozygous deletions in exons 7 and 8 of the SMN gene) and the control of mRNA concentrations in the future gene therapy of patients with SMA.
Owner:NGUYEN HUYNH MAI THI
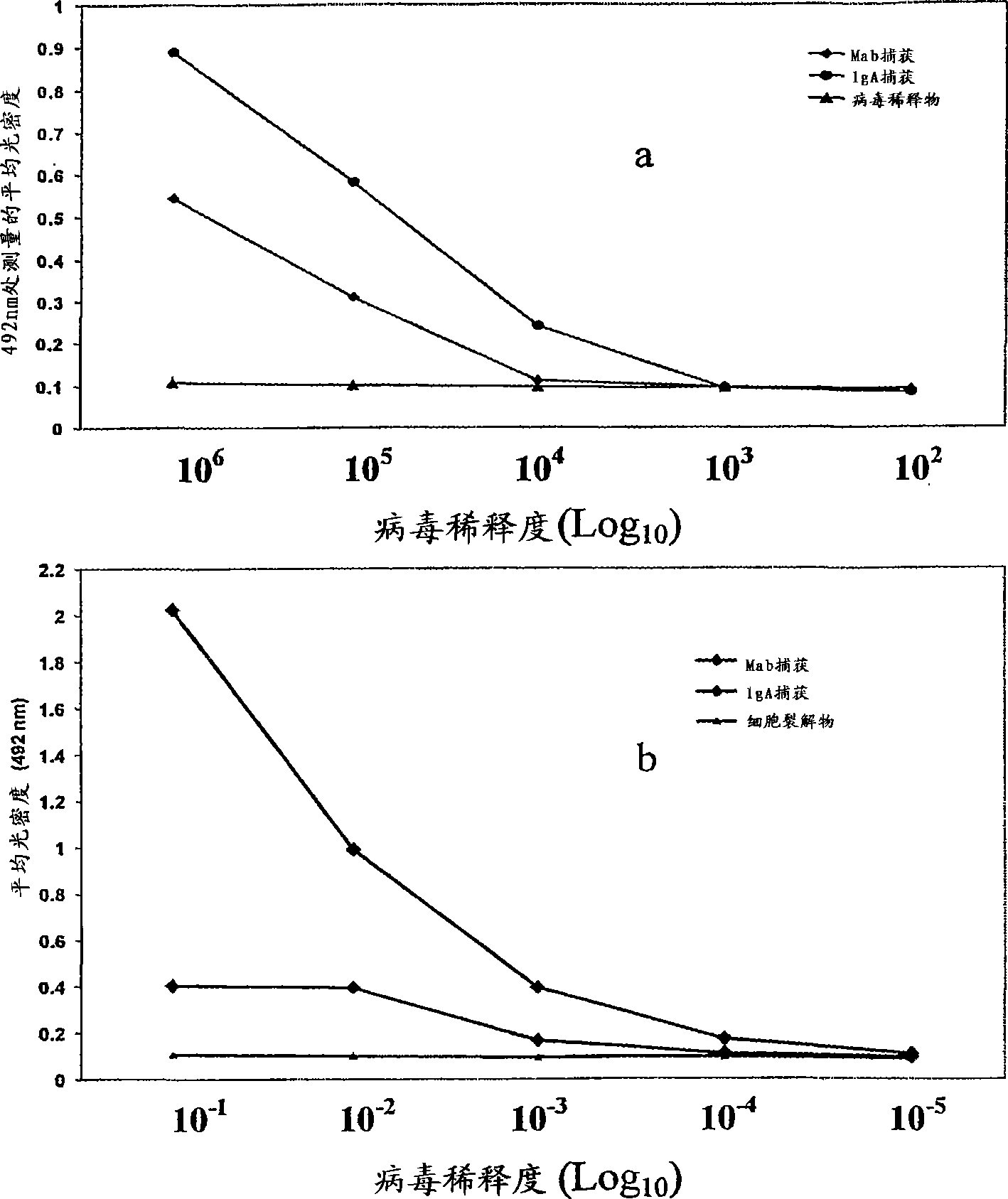
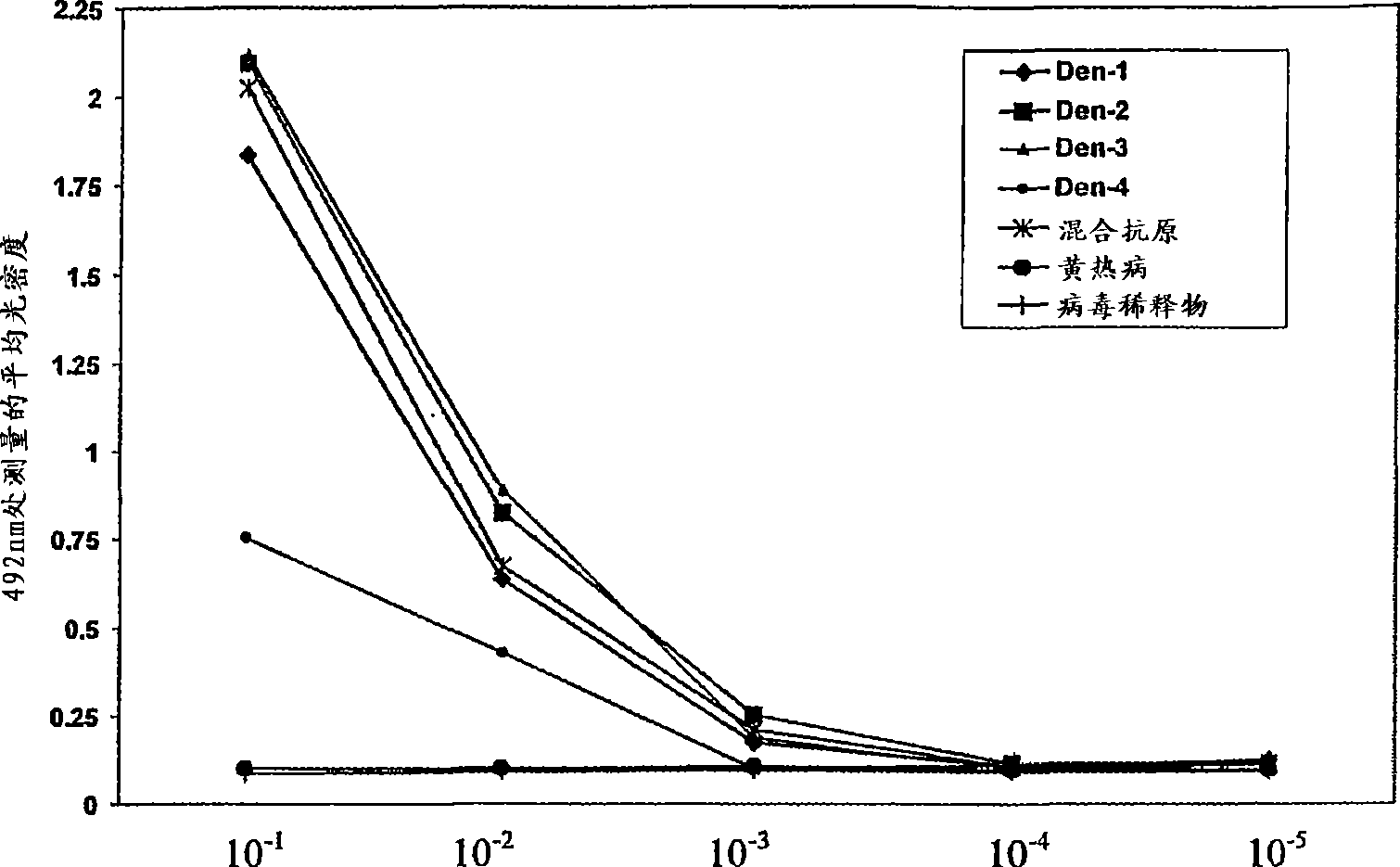
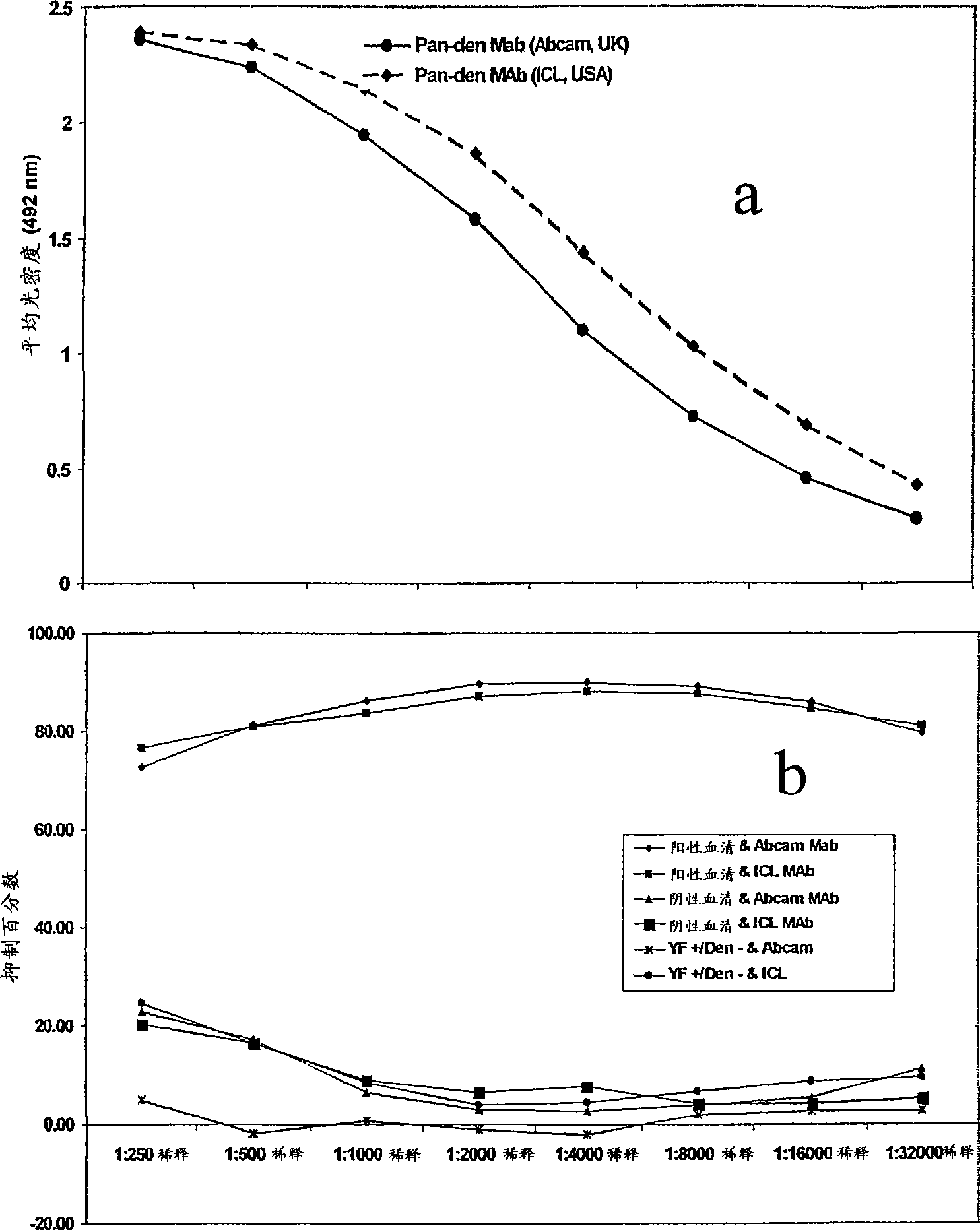
Competitive enzyme linked immunosorbent assay (C-ELISA) for the detection of a flavivirus specific antibody
InactiveCN101449165ASsRNA viruses positive-senseBiological material analysisSpecific immunitySecondary Infections
A competitive enzyme-linked immunosorbent assay (C-ELISA), using flavivirus member specific immunological agents was developed to detect antibody specific to members of the flaviviruses indicative of exposure to flavivirus. The test is based on a competition for epitope binding on the envelope protein of the flavivirus antigen captured using anti-flavivirus IgA in the presence of flavivirus positive serum. This test has comparable sensitivity specificity and speed to the virus neutralization assay (VNT). C-ELISA is a versatile technique, which could have various applications. Slight modifications of this protocol could lead to a C-ELISA-based detection method of secondary infection or one that could be used for serotype specific sero-epidemiological studies and / or vaccine evaluation. The protocol developed for C-ELISA was demonstrated using dengue lysate antigen and dengue specific monoclonal antibody. This can be used against other flaviviruses and the results for Japanese encephalitis illustrates this.
Owner:NATIONAL ENVIRONMENT AGENCY
Preparation method and application of carbendazim hapten and antigen
InactiveCN109265404AQuick checkRetain chemical structureOvalbuminSerum albuminEpitope2-aminobenzimidazole
The invention discloses a preparation method and application of a carbendazim hapten and antigen. The method is characterized in that the carbendazim hapten is prepared from 2-aminobenzimidazole and butanedioic anhydride which are reacted; the carbendazim antigen is obtained by coupling the carbendazim hapten and carrier protein. The antigen prepared through the method presents a specific carbendazim antigenic determinantantigen epitope, so that screening out a high-specificity carbendazim monoclonal antibody becomes possible; the generated antibody is high in specificity and high in sensitivity, and can be used for building an enzyme-linked immunosorbent assay method and a colloidal gold test strip rapid determination method, so that carbendazim in tobacco and food is fast detected.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +2
Enzyme-linked immunosorbant assay kit applicable for carbofuran residue analysis
The invention discloses a kind of enzyme linked immuno-sorbent assay reagent box applying to the analysis of the Carboruran residue, and it includes the box body, the 96-hole / 40-hole enzyme marker board placed in the box body and the reagent placed in the box body, and its characteristic lies in that in each hole of the enzyme marker board is the envelope antigen enveloped by the envelope liquid and able to combined and react with the anti-Carboruran antibody specioficity and sealed with 1.0-3.0% nonfat dried milk, and that the reagent in the box includes cleaning mixture, substrate-matter diluents, Carboruran standard solution, anti-CArboruran antibody, horseradish peroxide enzyme marker sheep anti-rabbit antibody, substrate matter, color-displaying matter and reaction-termiinated liquor.
Owner:ZHEJIANG UNIV
Method for the Preparation of Ready-to-Use Support for Rapid Enzyme-Linked Immunosorbent Assay (Elisa)
InactiveUS20080044928A1Quick and accurate and stable estimationRapid performance of methodMaterial analysisAntigenElisa kit
The present invention provides a method for the preparation of ready-to-use solid support for ELISA for rapid identification and quantitative estimation of protein / antigen in the test samples, and performances of the assay itself. The invention also provides for a quick, accurate and stable estimation of protein / antigen in the test samples. The invention also provides an ELISA kit comprising of ready-to-use solid support along with wash buffers, chemical substrate, substrate buffer, stock solution, and positive and negative control samples.
Owner:MAHARASHTRA HYBRID SEEDS CO LTD
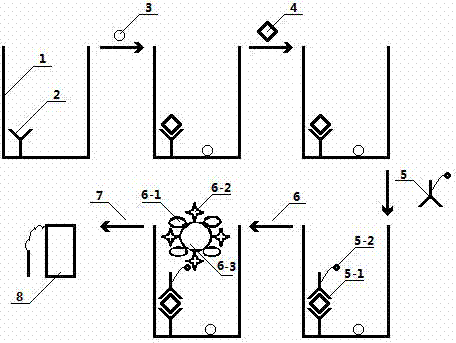
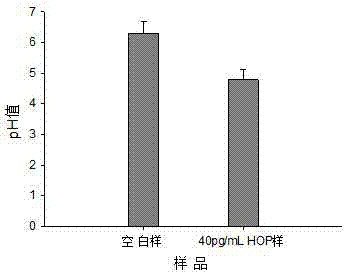
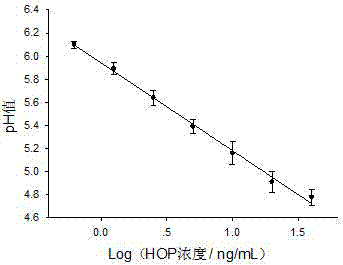
ELISA (enzyme-linked immuno sorbent assay) method based on pH (potential of hydrogen) meter
ActiveCN104849443AEasy to detectIncrease the amount of immobilizationBiological testingSaccharic acidSpecific immunity
The invention discloses an ELISA (enzyme-linked immuno sorbent assay) method based on a pH (potential of hydrogen) meter. The ELISA method comprises the following steps of performing antigen-antibody specificity immune reaction in an ELISA plate, capturing the antigen or antibody of the analyzing matter in a sample, introducing the glucose oxidase-glucose biological catalytic reaction, and using the pH meter to measure the pH value of the mixed product solution containing the glucose, wherein the size of the pH value is negatively related with the concentration of the analyzing matter in the sample, so as to complete the ELISA based on the pH meter. The ELISA method has the advantages that the signal is read by the low cost pH meter, so the cost of quantitative analysis is reduced; the response signal of single antigen-antibody bonding reaction is amplified by the micrometer or nanometer immobilized glucose oxidase; while the good specificity of the traditional ELISA method is maintained, the immobilizing amount of the glucose oxidase is increased by the micrometer or nanometer particles; the response signal of the single antigen-antibody specificity bonding reaction is amplified, and the detection sensitivity is improved.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Rapid heat - mediated method for enzyme - linked immunosorbent assay procedure
This invention relates to a rapid and efficient method for carrying out enzyme-linked immunosorbent assay for detection of minute quantities of biomolecules such as antigen, antibody etc. This invention particularly relates to heat-mediated immobilization of antigen or antibody on to the activated surface followed by performing subsequent steps of ELUSA by controlled temperature. The invented procedure has reduced the total time required for ELISA to around 3 h. The invented ELISA procedure is rapid, economical, reproducible and simple. The invented procedure is usefull for carrying out ELISA required in clinical diagnostics, molecular biology, agriculture, food technology, environmental science etc. The invented ELISA method is simple, time saving and obviates the time consuming procedure. This method has the potential for automation.
Owner:COUNCIL OF SCI & IND RES
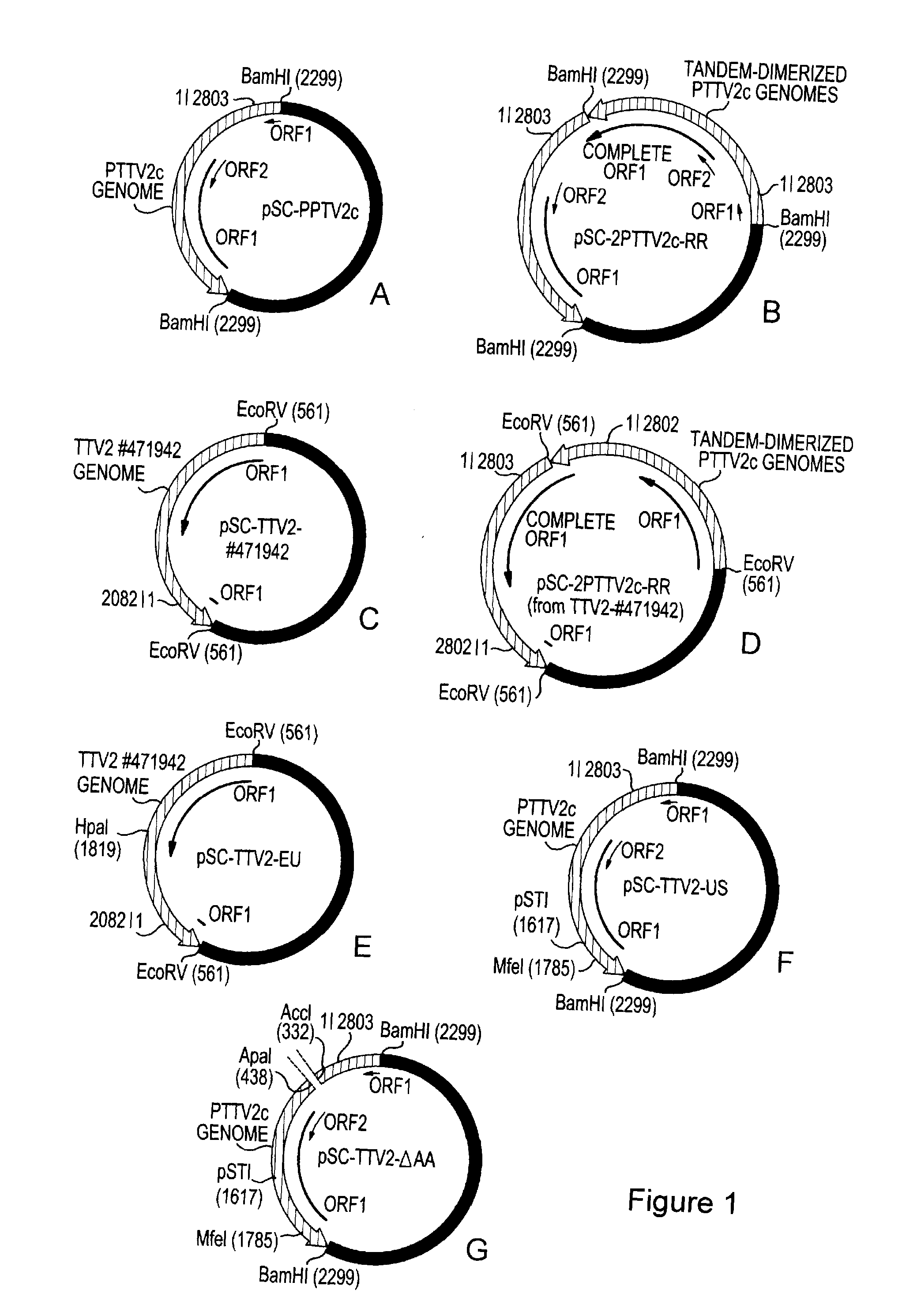
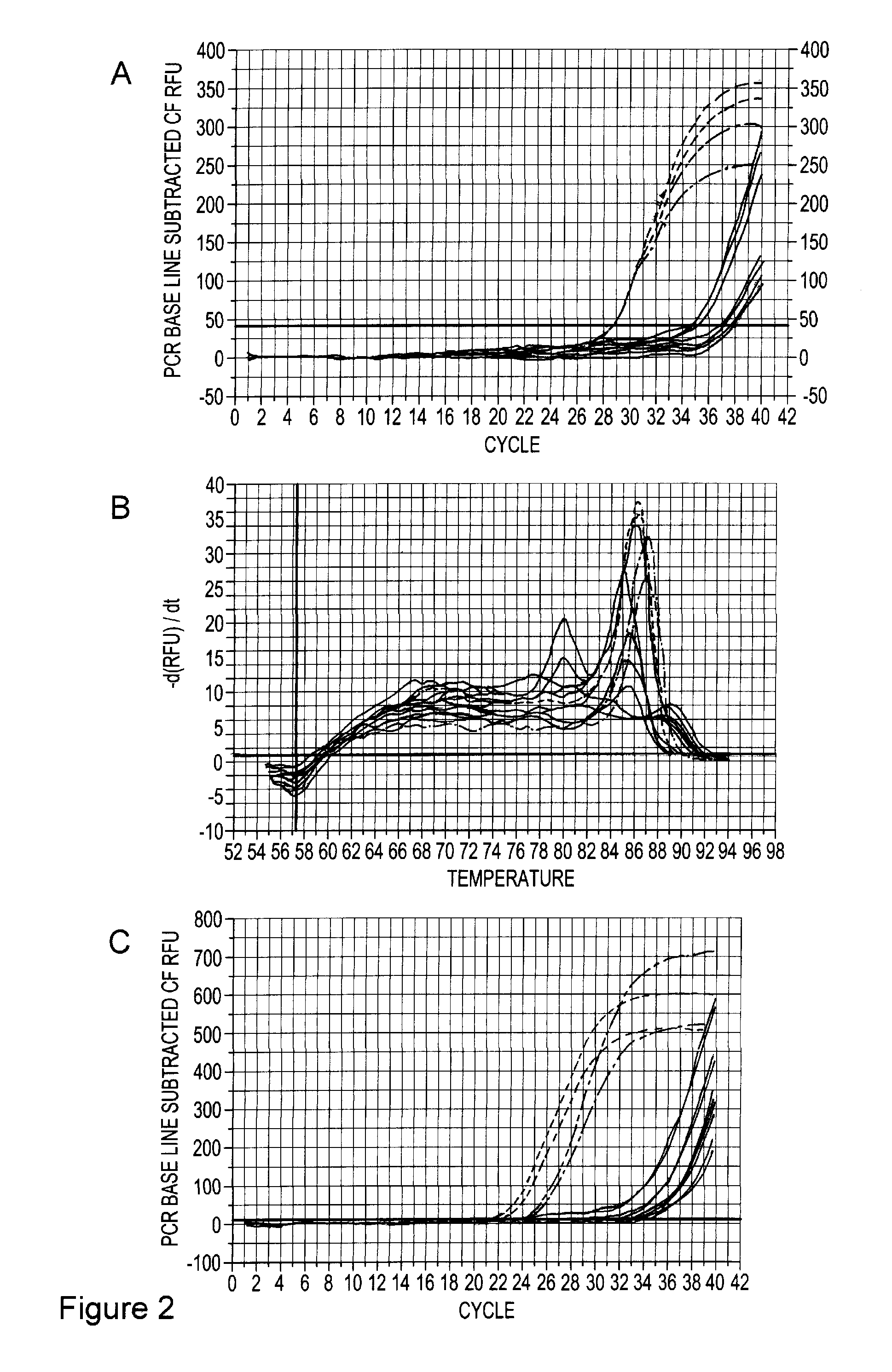
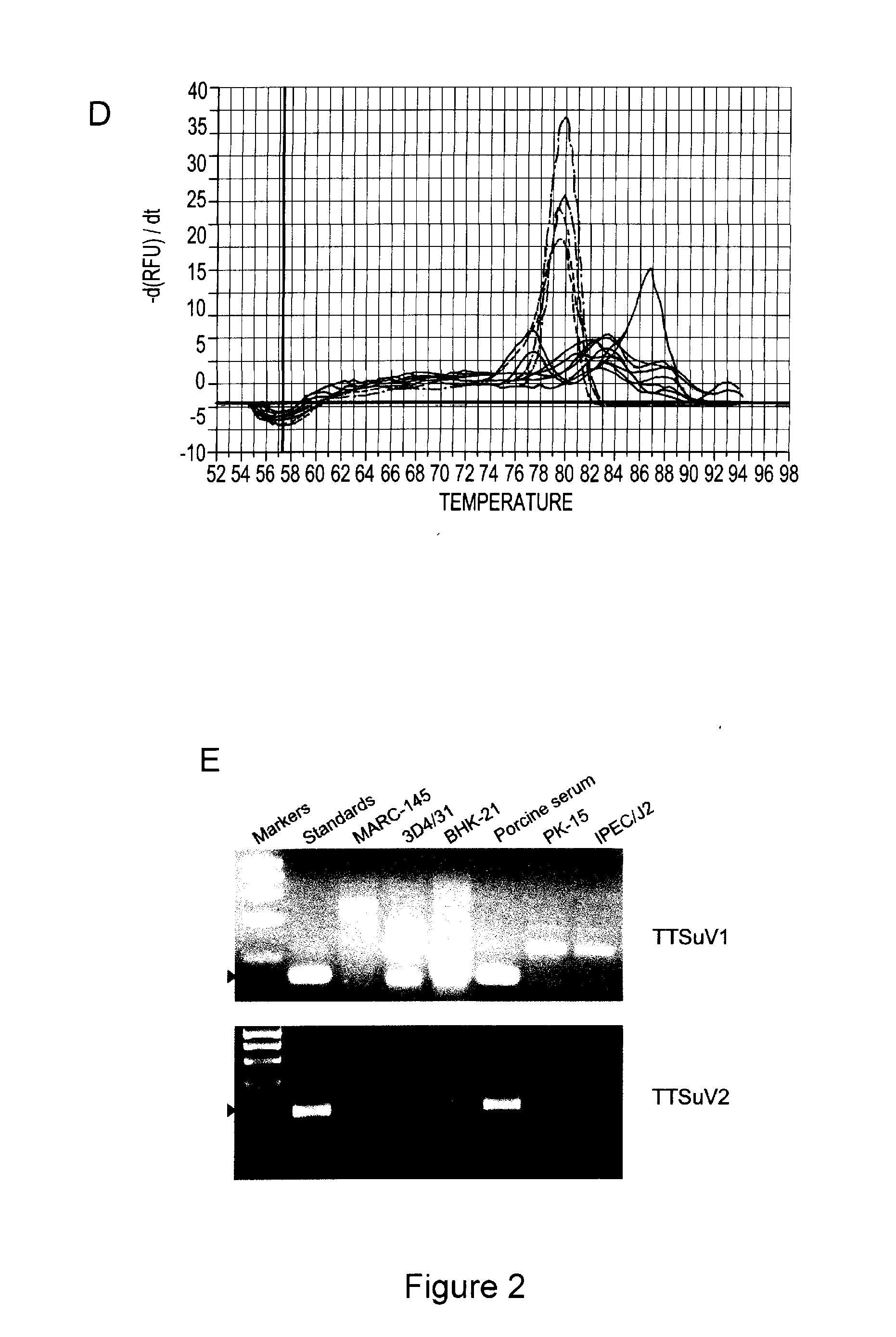
Infectious genomic DNA clone and serological profile of torque teno sus virus 1 and 2
The present invention also provides infectious DNA clones, biologically functional plasmid or viral vector containing the infectious nucleic acid genome molecule of Torque teno sus virus (TTsuV). The present invention also provides methods for diagnosing TTsuV infection via immunological methods, e.g., enzyme-linked immunoabsorbent assay (ELISA) and Western blot using PTTV specific antigens for detecting serum PTTV specific antibodies which indicate infections TTsuV1, TTsuV2, and individual TTsuV1 genotypes.
Owner:VIRGINIA TECH INTPROP INC
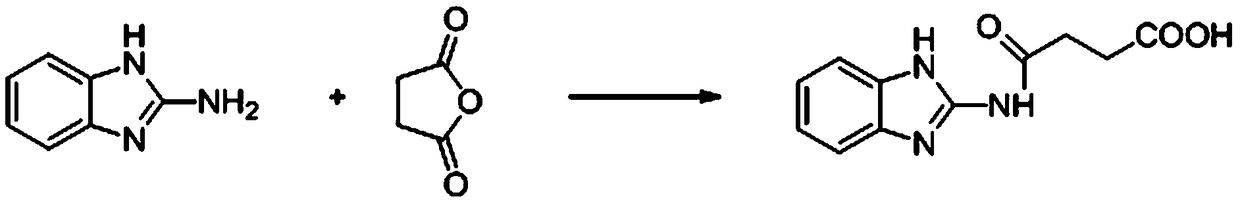
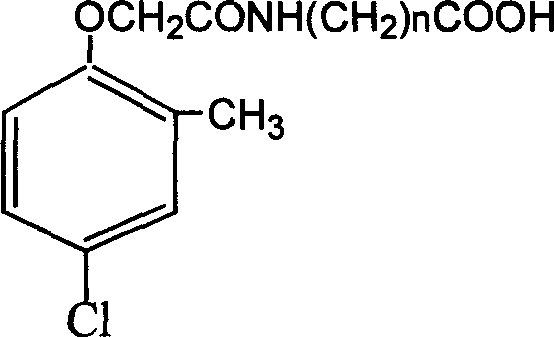
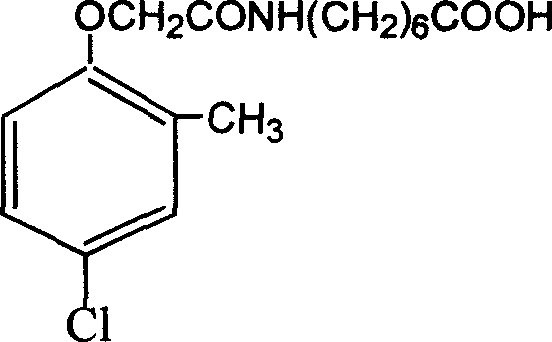
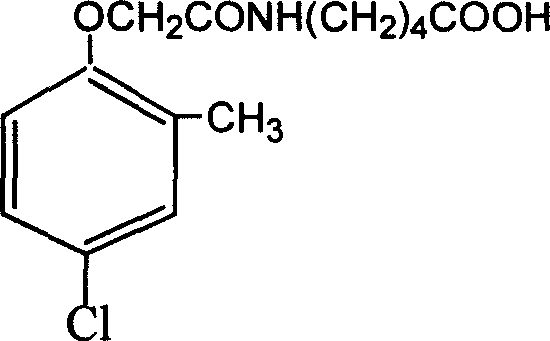
Method for preparing 2 metho 4 chlorine artificial half antigen, artificial antigen, specific antibody and application
InactiveCN1562963AFast and accurate analysis and detectionEasy to handleImmunoglobulinsCarrier-bound/immobilised peptidesCarboxylic acidSpecific antibody
Raw material-2-methyl-4-chlorophenoxy carboxylic acid (2-ME-4-CHL) is acrylated by sulfoxide chloride, then reacting with amino-butyric and amino-caproic acid respectively, to prepare half antigens: 4-(2-methyl-4-chlorophenoxyacetyl) amino butyric acid and 6-(2-methyl-4-chlorophenoxyacetyl) amino caproic acid (MB and MC for short, resp). And 2-ME-A-CHL itself can be half-antigen (M for short). Halfantigens M, MB and MC are coupled with protein to produce immune antigen and envelope antigen by carbodi-imide and mixed acid anhydride methods. Immue antigen immune animals are used to produce special antibody with high avidity to 2-ME-4-CHL. This antibody does not react with other compounds. Enzyme-linked immunosorbant assay established with said antibody can test trace ammount of 2-ME-4-CHL residues quickly and conveniently.
Owner:ZHEJIANG UNIV
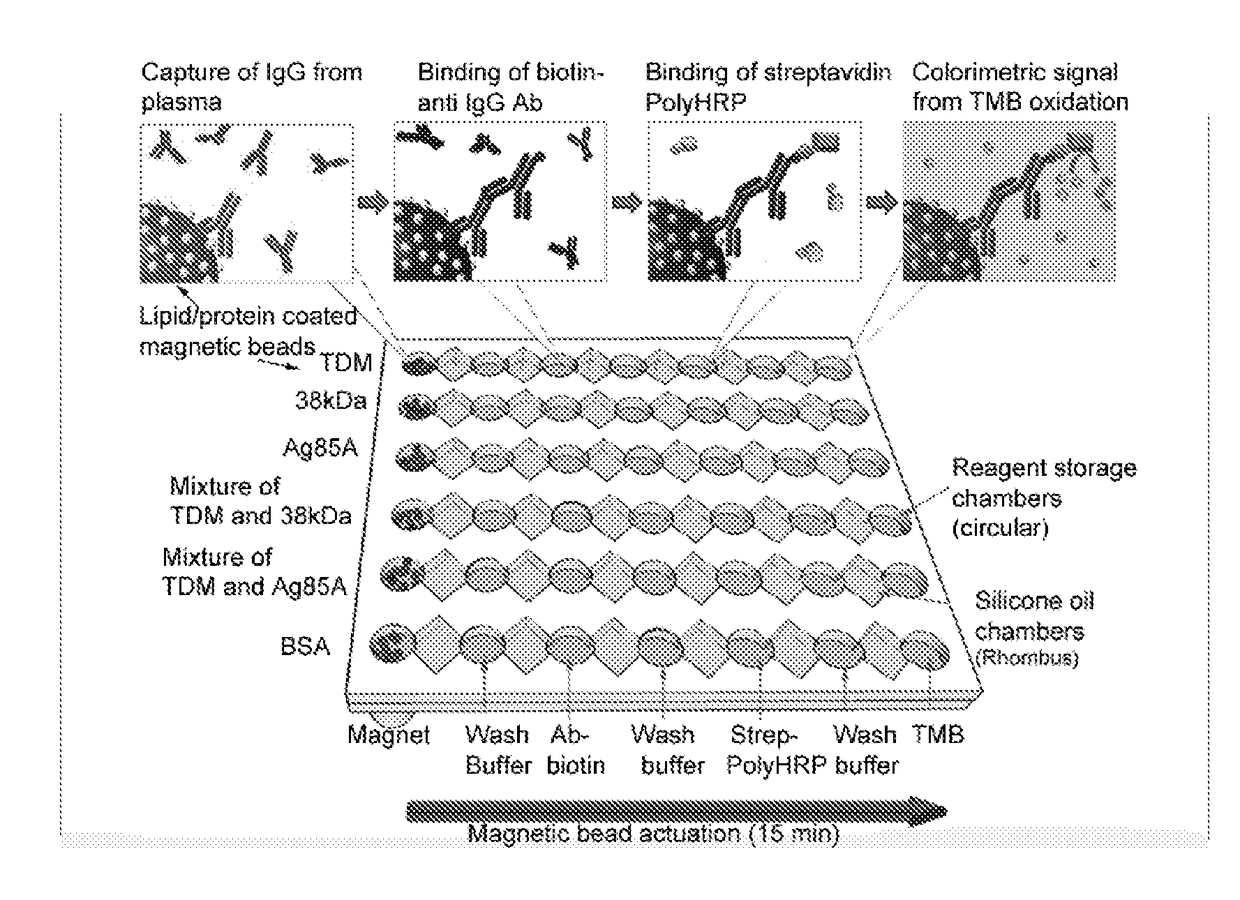
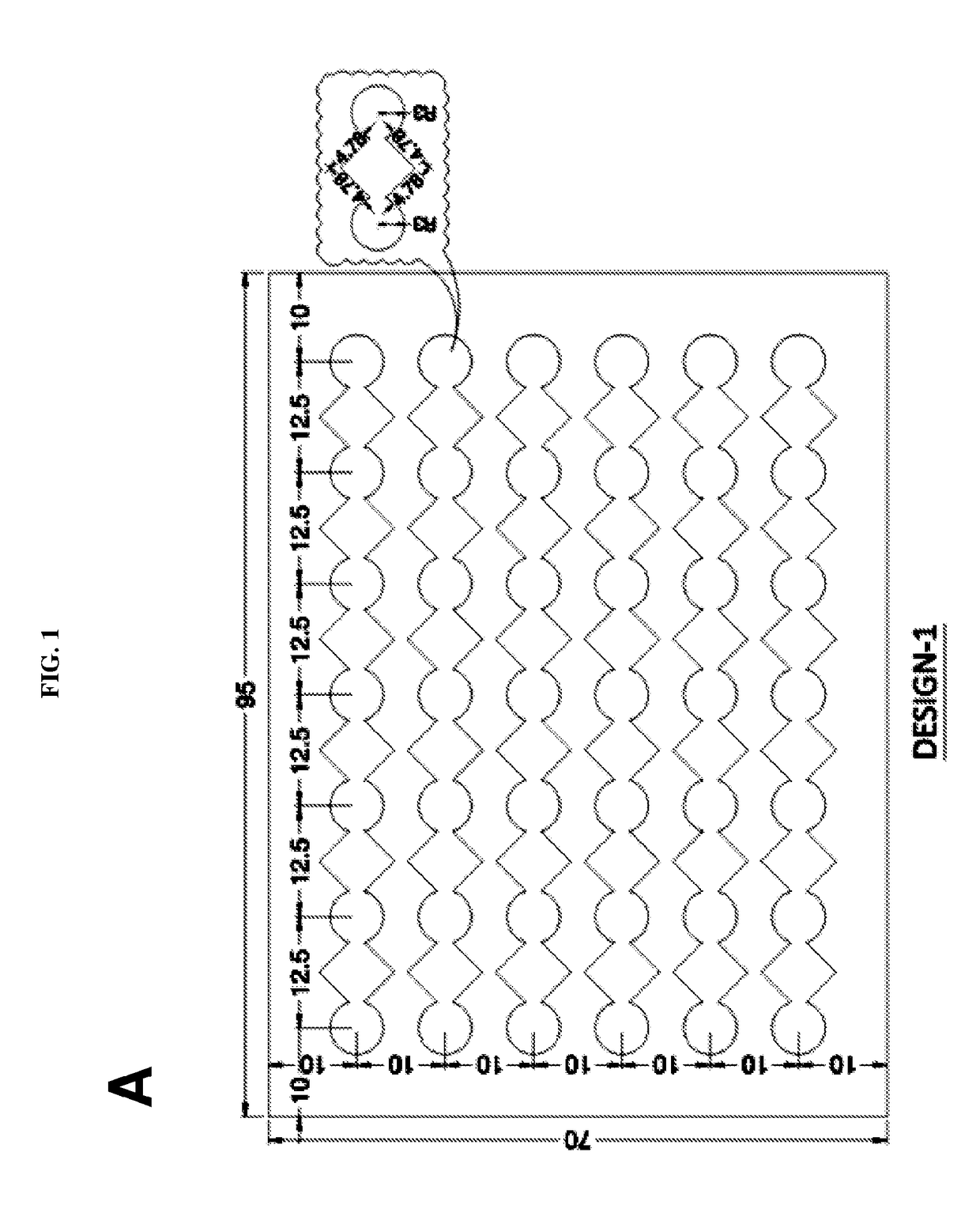
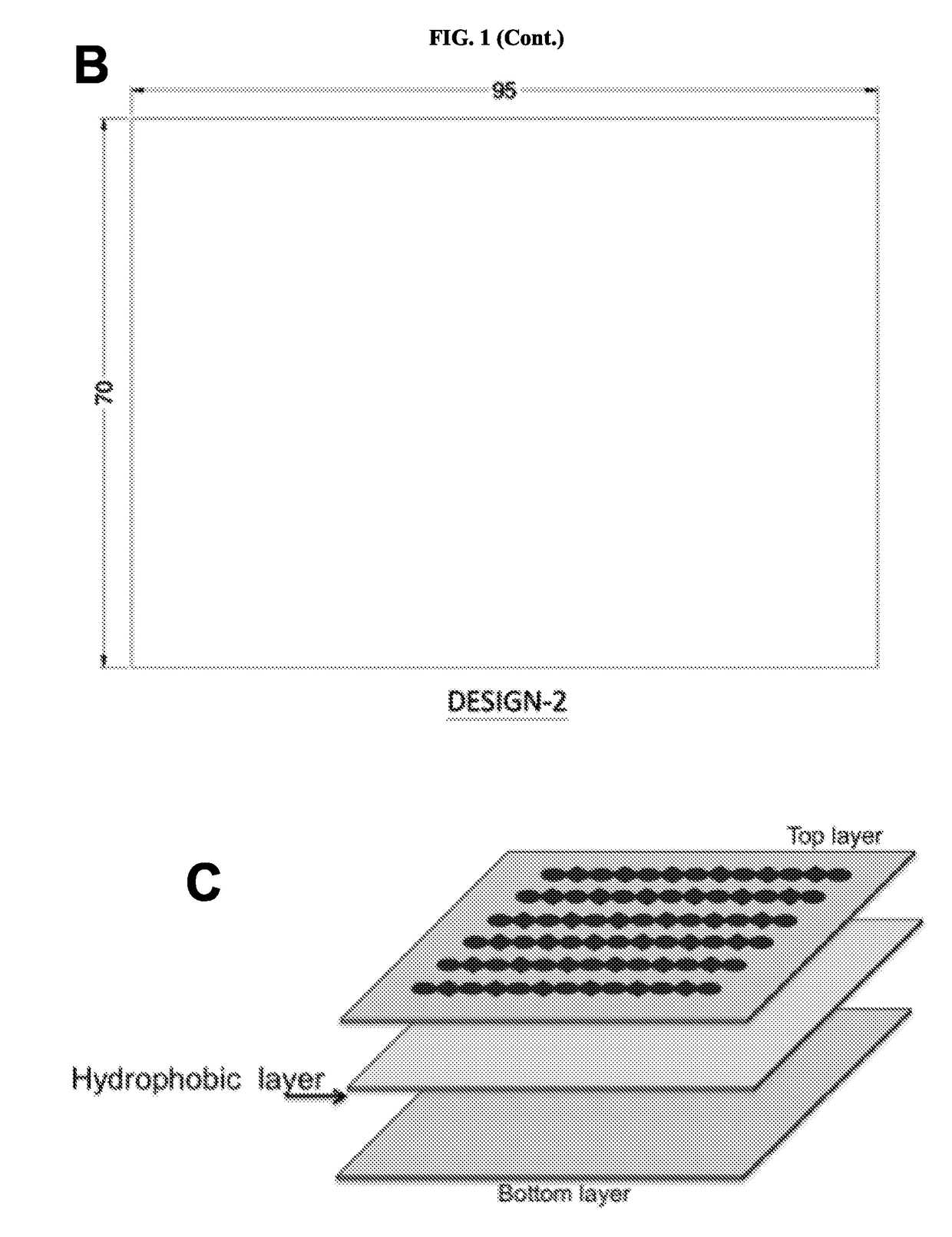
Novel design of enzyme-linked immunosorbent assay plates and systems and methods of use thereof
The present disclosure refers to an enzyme-linked immunosorbent assay (ELISA) plate comprising at least one row of reaction chambers, wherein the reaction chambers in the same row are in fluid communication with each other. Also enclosed is a system for detecting one or more target analytes comprising an ELISA plate as described herein, a plurality of magnetic beads and a magnet configured to cooperate with the magnetic beads. Also encompassed is a method of performing an ELISA assay which comprises of moving magnetic beads through subsequent reaction chambers, wherein the reaction chambers are alternatingly filled with a non-aqueous liquid, such as silicone oil, and aqueous ELISA reagents.
Owner:AGENCY FOR SCI TECH & RES
A neuroglobin enzyme-linked immunosorbent assay kit and the use of it
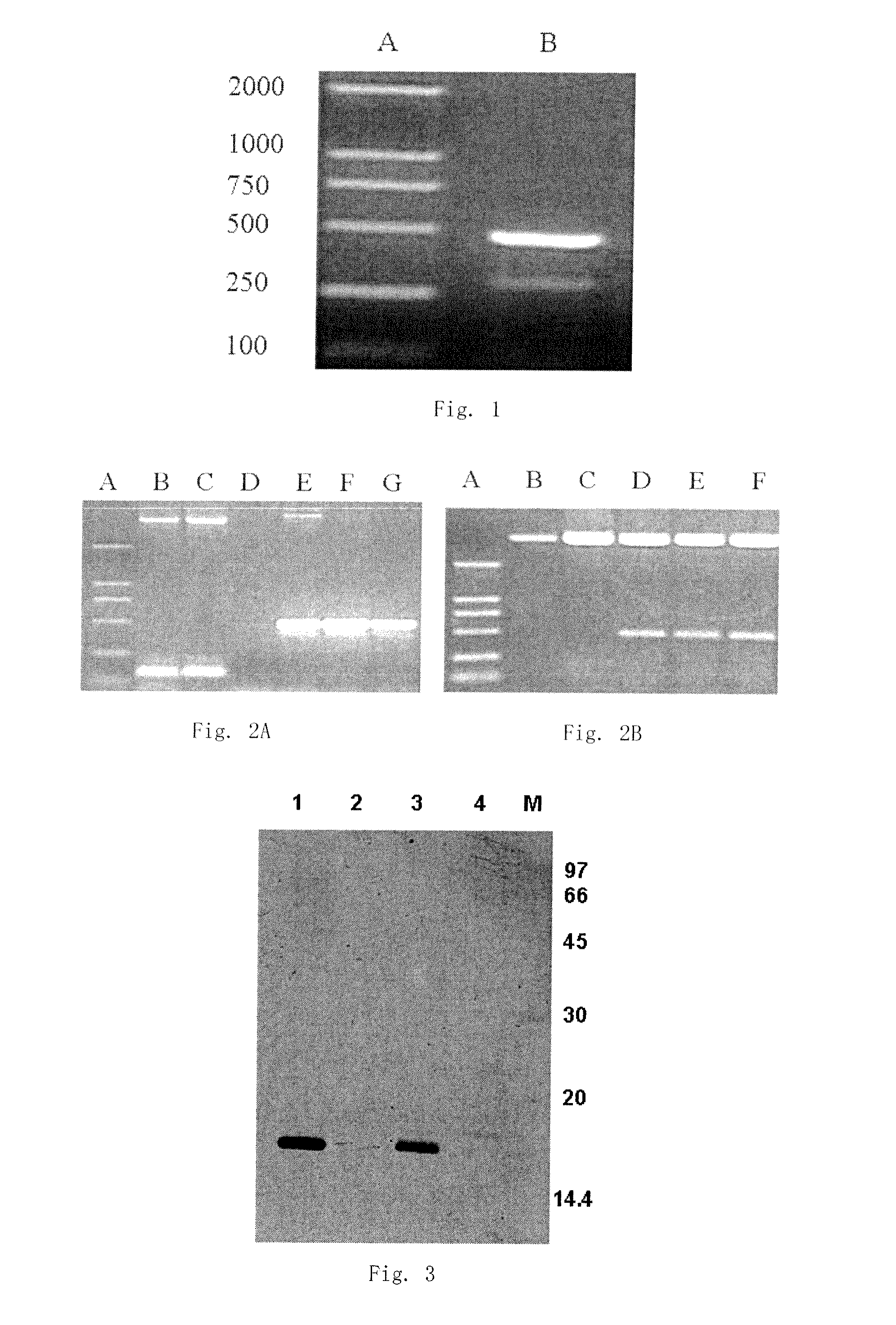
InactiveUS20100028917A1Strong specificityHigh sensitivityDisease diagnosisBiological testingSerum igeProtein.monoclonal
The invention discloses a Neuroglobin enzyme-linked immunosorbent assay kit and the use of it. The Neuroglobin enzyme-linked immunosorbent assay kit provided by the invention includes Neuroglobin polyclonal antibody, Neuroglobin monoclonal antibody and enzyme labeled antibody. Neuroglobin monoclonal antibody is produced through culturing hybridoma cells prepared by cell conjugation after inoculating mice with Neuroglobin antigen, whereas Neuroglobin polyclonal antibody is produced from animal antiserum after inoculating with Neuroglobin antigen.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA +1
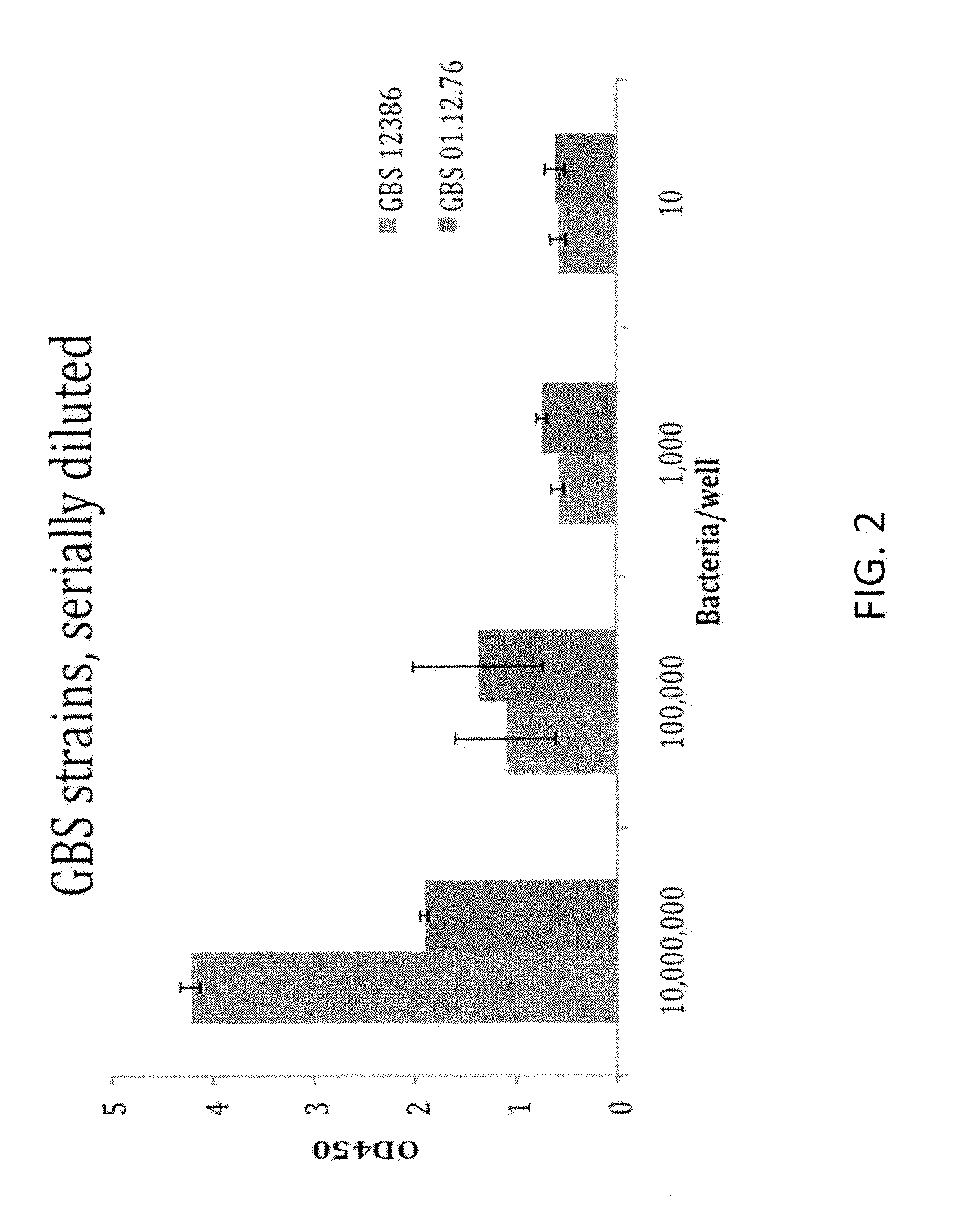
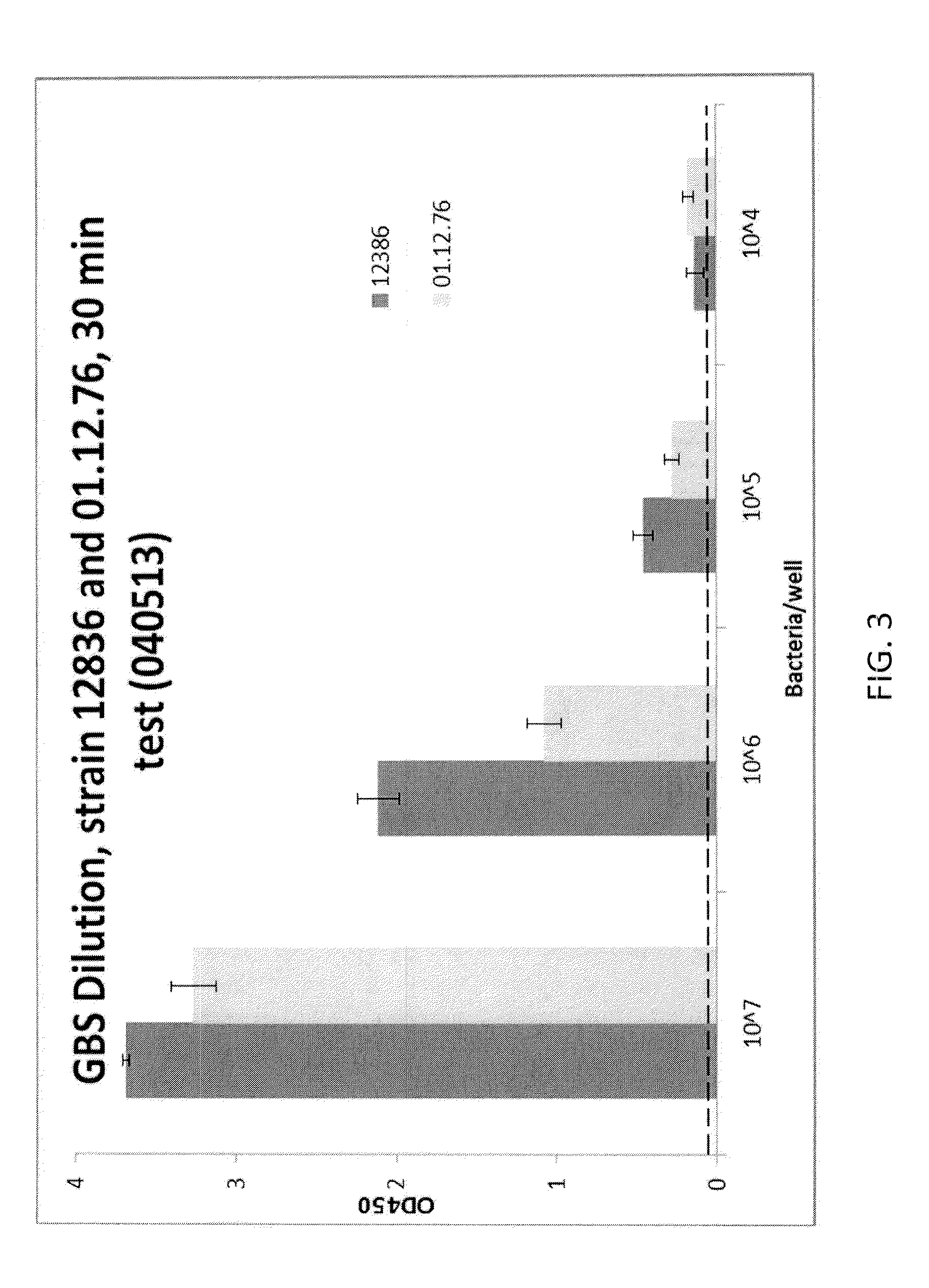
Rapid Enzyme-Linked Immunosorbant Assay for Detection and Identification of Pathogens and Determination of Antimicrobial Susceptibility
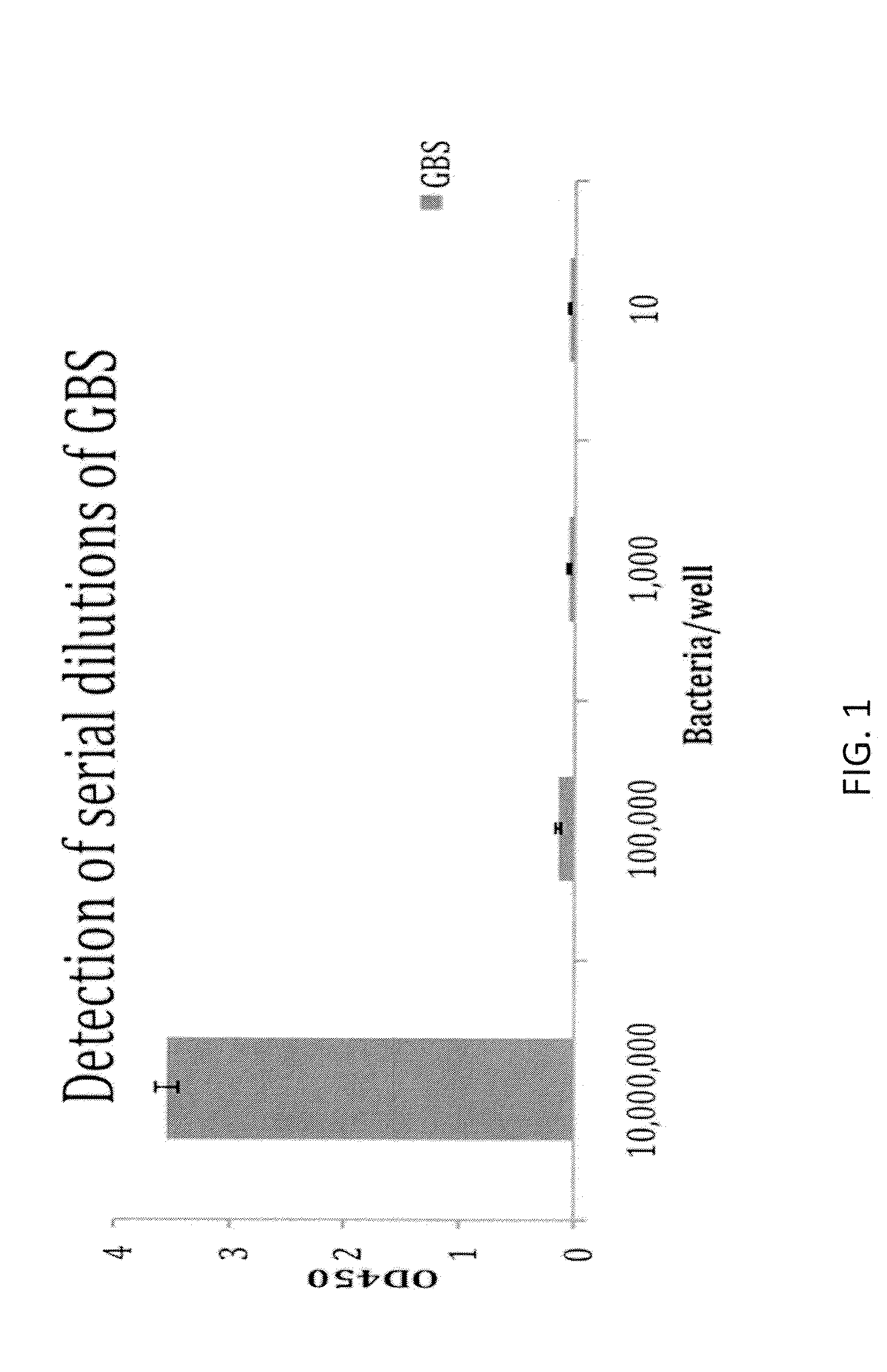
ActiveUS20140315219A1High sensitivityStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsNon targetedNon target
Owner:BARNHIZER BRET T +1
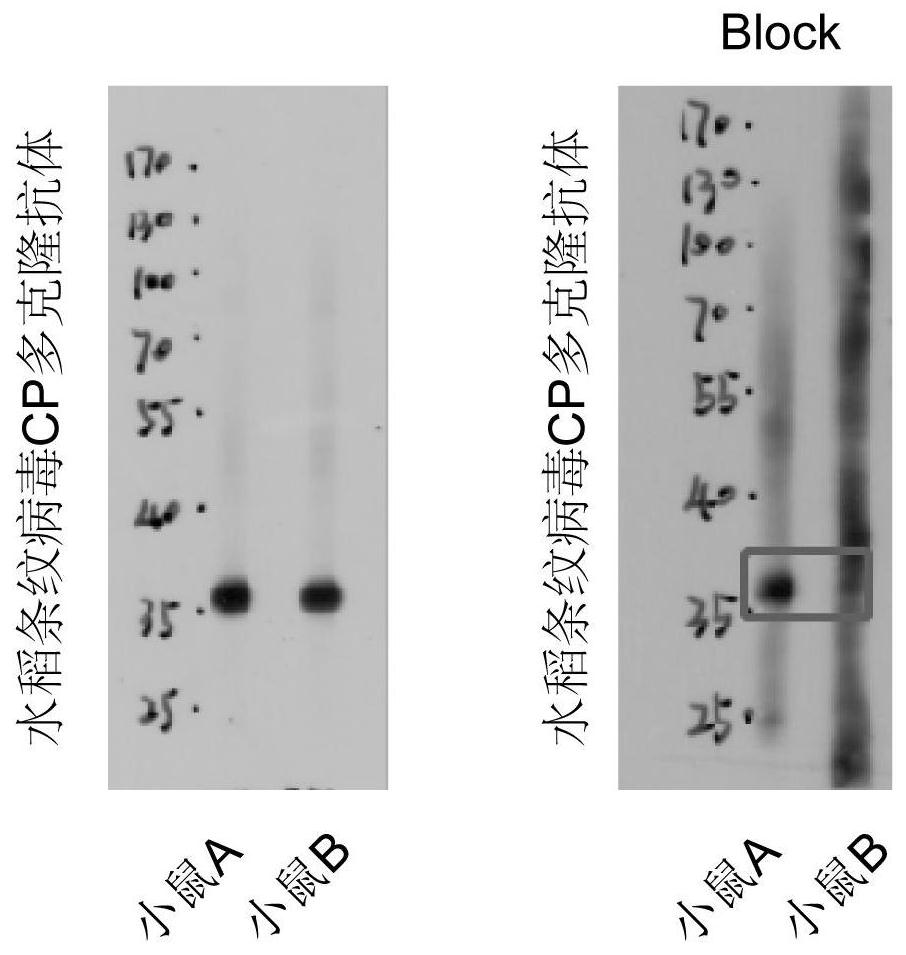
Polyclonal antibody of rice stripe virus nucleocapsid protein as well as preparation method and application thereof
ActiveCN111606979AQuick checkEfficient detectionSsRNA viruses negative-senseBacteriaDisease monitoringIMMUNE FLUORESCENCE
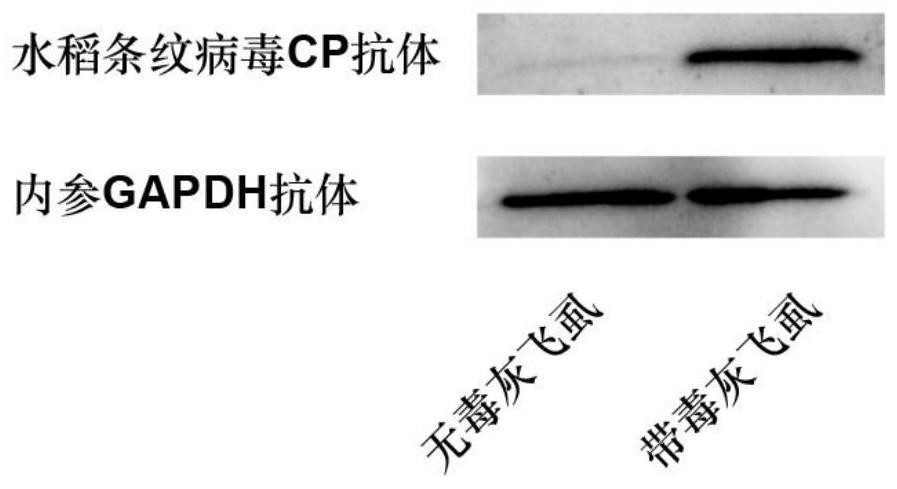
The invention provides a preparation method and application of a polyclonal antibody of a rice stripe virus nucleocapsid protein, and belongs to the technical field of rice stripe virus detection. Thepolyclonal antibody of the rice stripe virus nucleocapsid protein CP is obtained by immunizing animals with the rice stripe virus nucleocapsid protein CP as immunogen; and the amino acid sequence ofthe rice stripe virus nucleocapsid protein CP is as shown in SEQ ID No. 1. The polyclonal antibody of the rice stripe virus nucleocapsid protein has the characteristics of low cost, wide application range, multiple audiences, high sensitivity, and the like, and can be used for multiple biological detection experiments such as an immune spot determination method, an enzyme-linked immunosorbent assay method, a protein immunoblotting method, an immuno-fluorescence method, an immuno-electron microscopy method, and the like. By utilizing the disclosed polyclonal antibody, disease early warning is carried out through small brown rice plant hoppers at the beginning of outbreak of rice stripe disease, and multi-means disease monitoring is facilitated.
Owner:YANGZHOU UNIV
Artificial antigen, antibody of fenvalerate and uses thereof
The invention discloses a fenvalerate artificial antigen, with the molecular structural formula is as formulaó±, n=1-5, employing formula ó� as partial antigen, covalent coupling with protein for synthesis; the combination ratio between the partial antige and protein is 5:1-100:1. The invention also discloses the monoclonal or polyclonal immune globulin G which is got from immunizating mouse or rabbit by the above said fenvalerate artificial antigen and can occur specific immunological reaction with fenvalerate, the said fenvalerate specific antibody can be used to check the residual quantity of fenvalerate in sample. The invention also discloses a detecting agent box of direct and indirect competeing enzyme and immunoadsorption for fenvalerate residual analysis. The got antibody is used to detect fenvalerate by ELISA method, with the lowest detecting limit being 4.0í‚1.5 ug / l (0.004ppm), and the detecting sensitivity is high.
Owner:ZHEJIANG UNIV
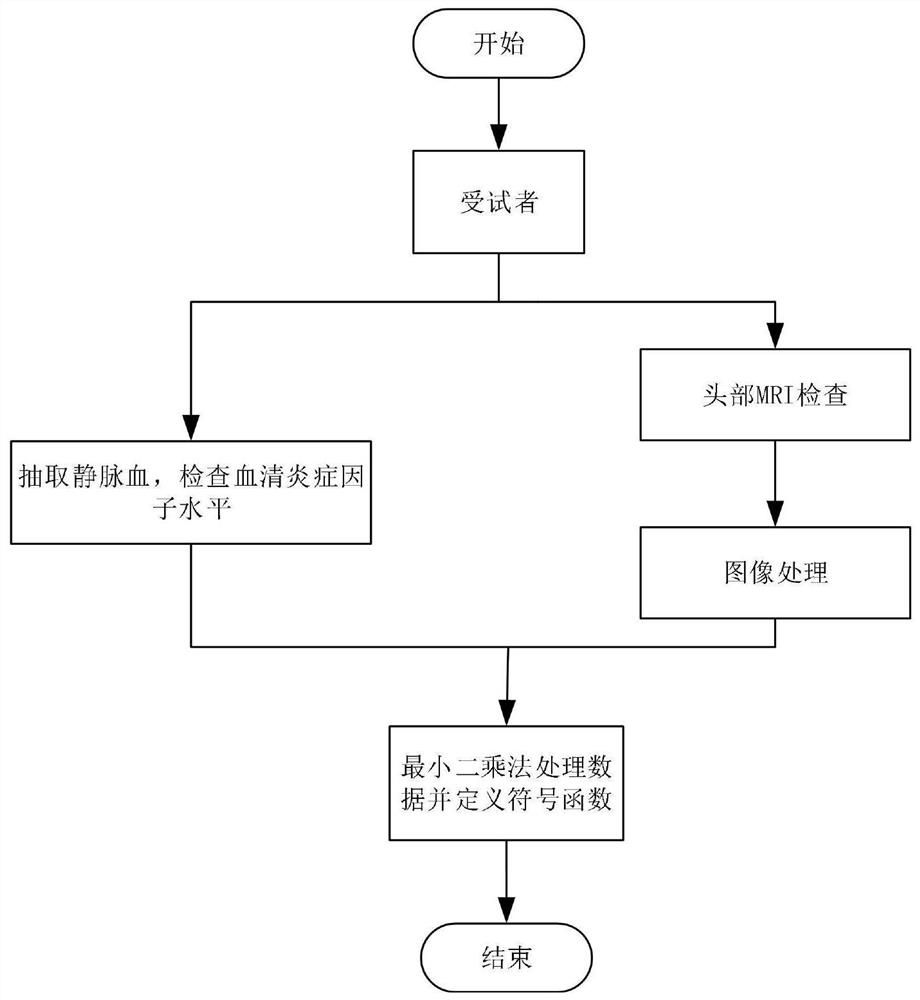
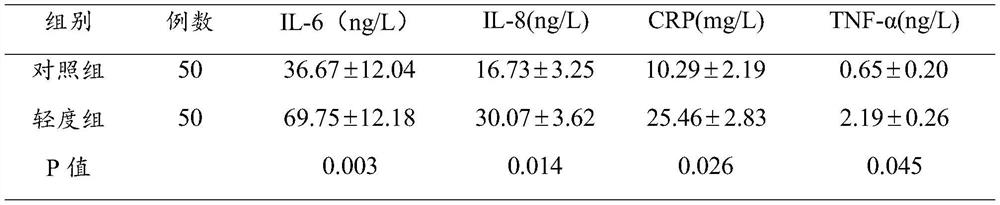
Brain injury marker analysis system based on DTI and serum factors
The invention relates to a brain injury marker analysis system based on diffusion tensor imaging (DTI) and a serum factor, the system comprises a DTI scanning module, a serum detection module and a data processing module, and the system is controlled according to the following modes: firstly, detecting the serum inflammatory factor level of venous blood extracted from an empty stomach by adopting an enzyme-linked immunosorbent assay; then, carrying out DTI scanning, carrying out image processing and data analysis on the collected images, and obtaining change data of the FA and the ADC; normalizing a serum inflammatory factor level parameter and an FA value and an ADC value obtained by magnetic resonance diffusion imaging by using a least square method, then defining a sign function, and taking a result of the sign function as a reference for a doctor to analyze and formulate a next detection scheme.
Owner:YANSHAN UNIV
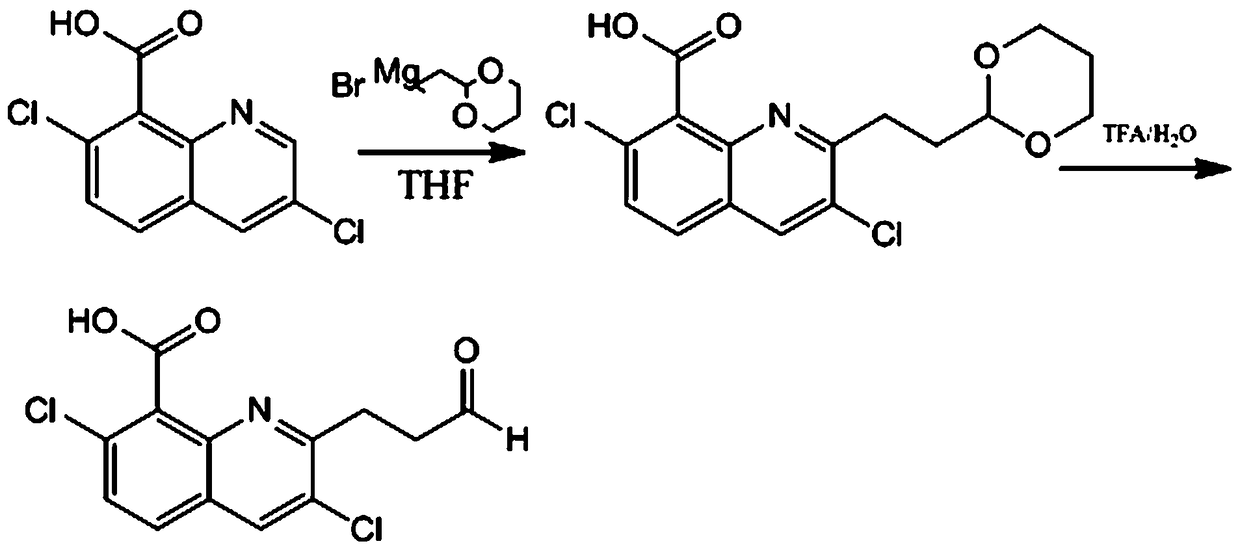
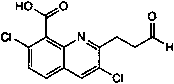
Preparation method and application of quinclorac hapten and antigen
The invention discloses a preparation method and application of a quinclorac hapten and antigen. The method is characterized in that the quinclorac hapten is prepared by using quinclorac and (1,3-dioxan-2-ylethyl)magnesium bromide for reaction to generate aldolactal quinclorac which is then reacted with trifluoroacetic acid; the quinclorac antigen is obtained by coupling the quinclorac hapten andcarrier protein. The antigen prepared through the method presents a specific quinclorac antigenic determinantantigen epitope, so that screening out a high-specificity quinclorac monoclonal antibody becomes possible; the generated antibody is high in specificity and high in sensitivity, and can be used for building an enzyme-linked immunosorbent assay method and a colloidal gold test strip rapid determination method, so that quinclorac in food and tobacco-planting soil is fast detected.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +2
Assay Analysis, Tracking and Reporting System (AATR)
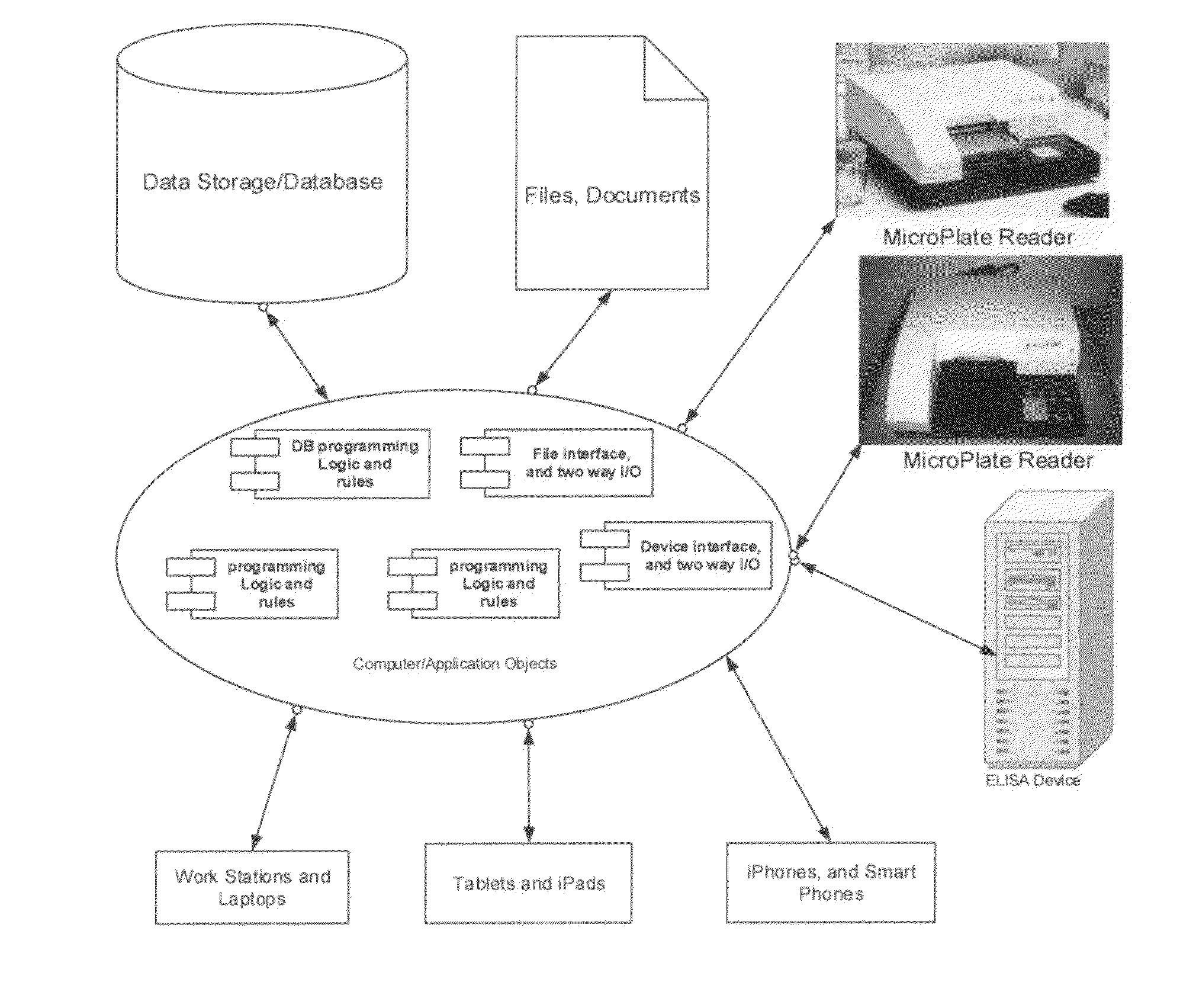
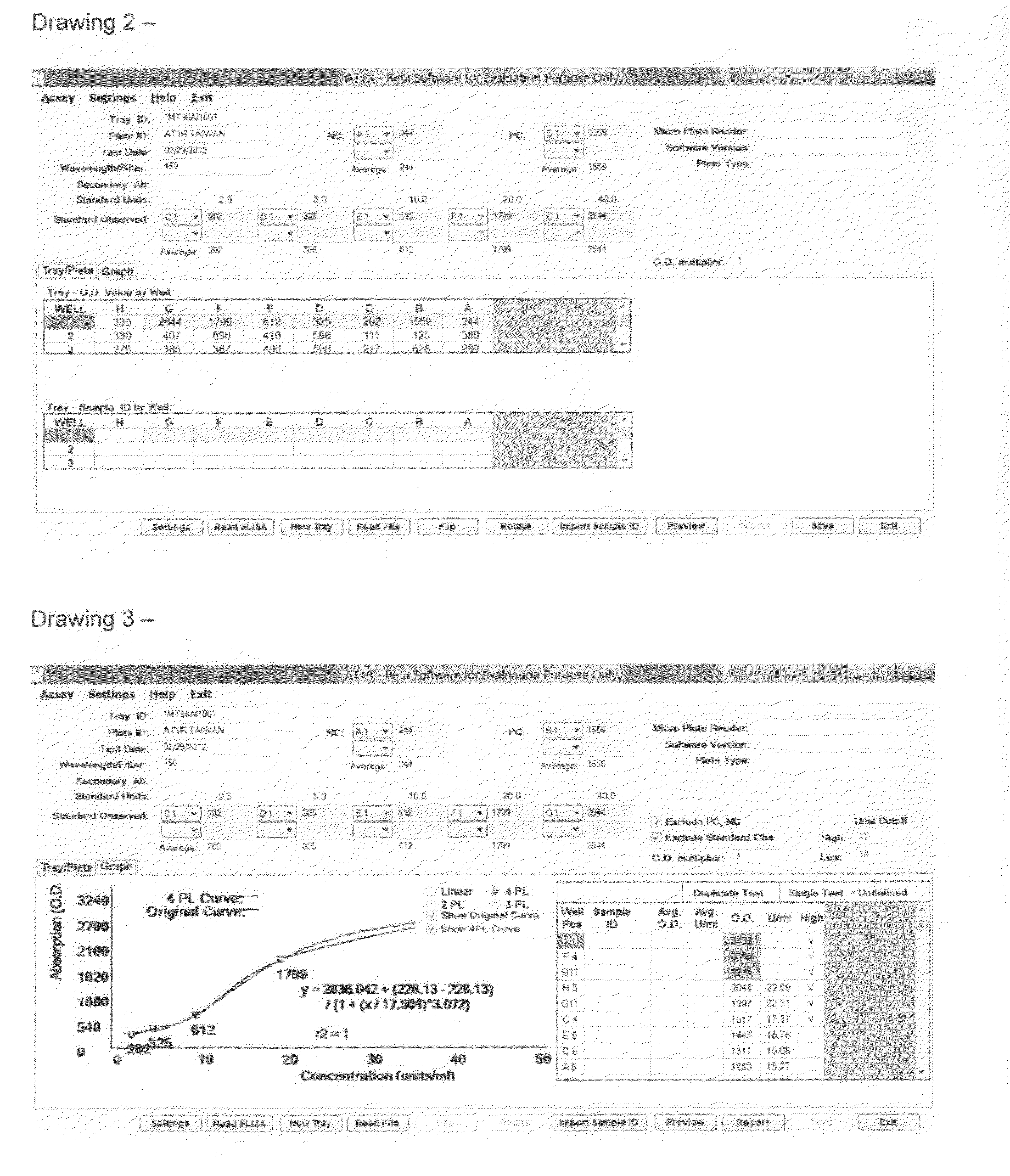
The Assay Analysis, Tracking and Reporting (AATR) is a software system to acquire and analyse Enzyme-Linked ImmunoSorbant Assays (ELISA) assays for the purspose of Antibody Screening, Detection, Concentration, Tracking, Dose Respone, Adsorption Analysis and Reporting using second / third / fourth degree polynomial, 4 and 5 Parameter Logistics best curve fitting regression analysis. AATR provides mechanism and capabilities to acquire data directly from Microplate Reader devices including batch mode and allows for import / export of ELISA data and sample ids for the tray. The regression analysis uses positive controls and negative controls and multiple standards data points. An average is calcuated for the duplicate samples. The software automatcialy color codes the wells and sample ids in the plate for the outliers data. Additional color coding and marker are used to designate the wells / samples above and below the highest and lowest thresold. AATR allows exporting of graphs, data points and reports in various formats.
Owner:JHA AAKASH +1
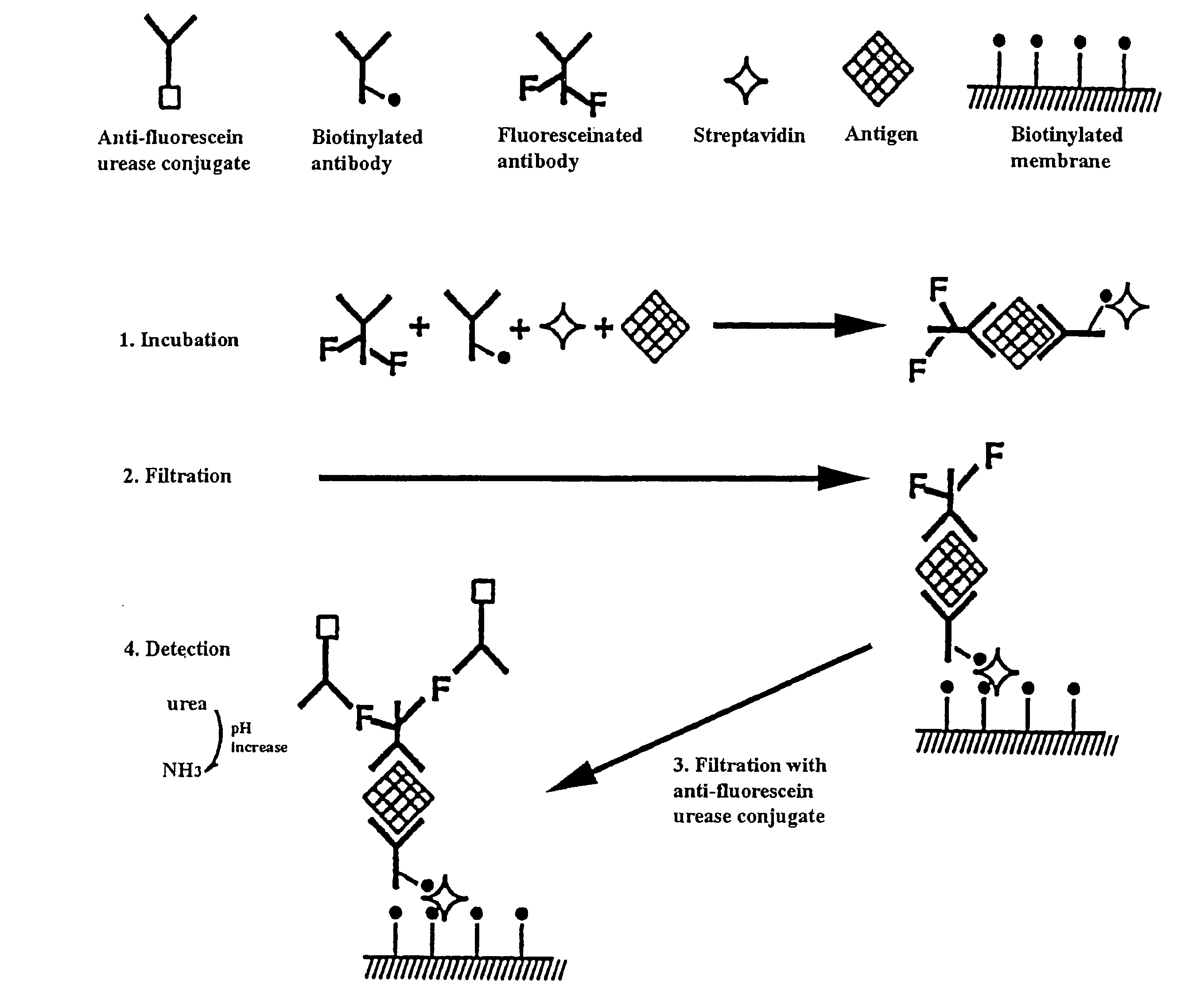
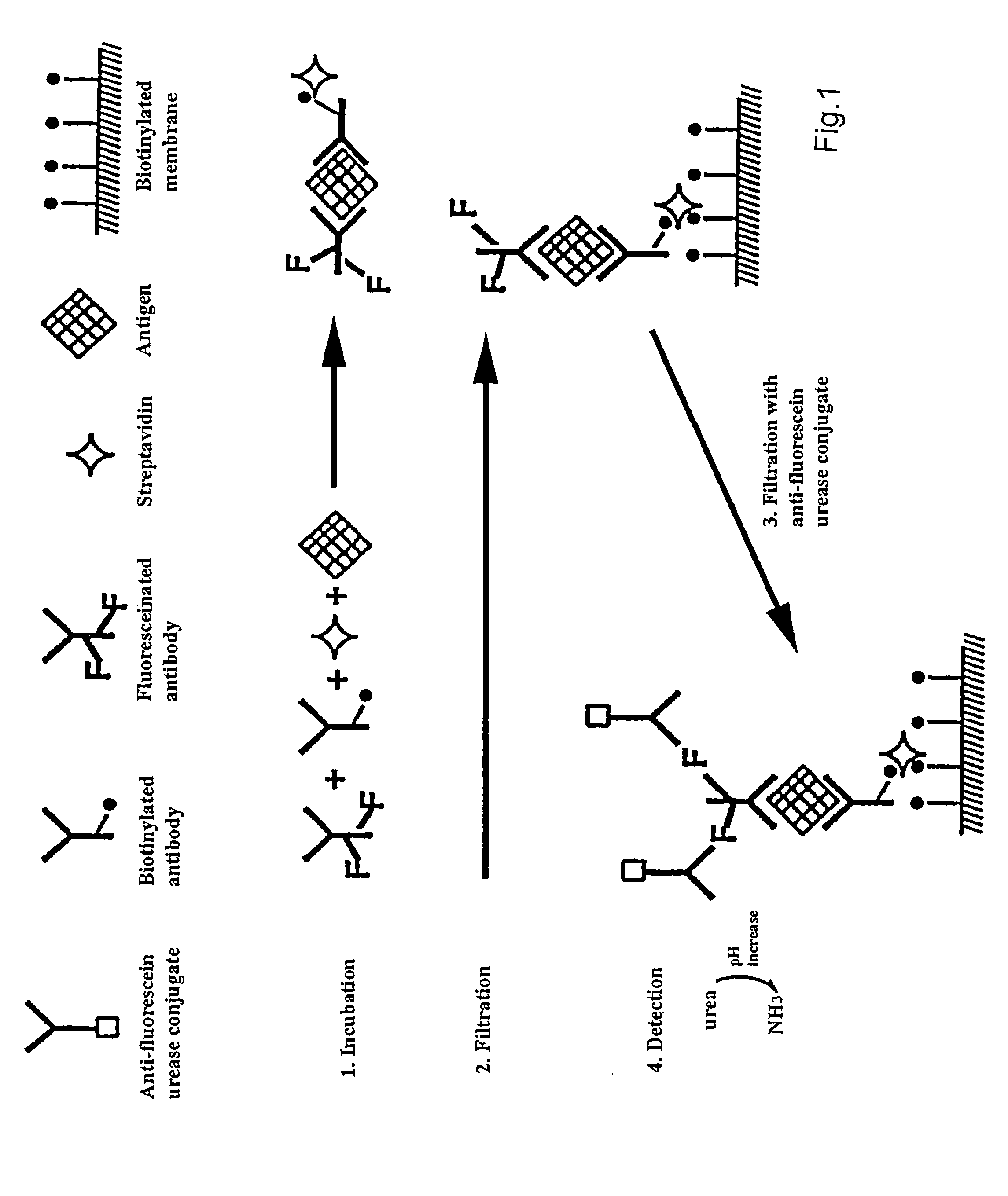
Genetically biotinylated recombinant antibody in immunofiltration assay by light addressable potentiometric sensor for identification of Venezuelan equine encephalitis virus
InactiveUS7309566B2Microbiological testing/measurementImmunoglobulins against virusesBiotin-streptavidin complexFiltration
A genetically biotinylated single chain fragment variable (scFv) antibody against Venezuelan equine encephalitis virus (VEE) being applied in a system consisting of an immunofiltration-enzyme assay (IFA) with a light addressable potentiometric sensor (LAPS) for the rapid identification of VEE is disclosed. The IFA entails formation of an immunocomplex sandwich consisting of VEE, biotinylated antibody, fluoresceinated antibody and streptavidin, capturing the sandwich by filtration on biotinylated membrane, and detecting the sandwich by anti-fluorescein urease conjugate. The concentration ratio of biotinylated to fluoresceinated antibodies is investigated and optimized. The IFA / LAPS assay sensitivity was approximately equal to that of a conventional enzyme-linked immunosorbant assay utilizing polystyrene plates and a chromogenic substrate, however, less time and effort were required for performance of the IFA / LAPS assay.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com