Rolling circle amplification DNA enzyme and covalent organic framework-based electrochemical-ELISA immunosensor
A covalent organic framework and immunosensor technology, applied in the field of electrochemical detection, can solve the problems of high cost and easy inactivation of biological enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
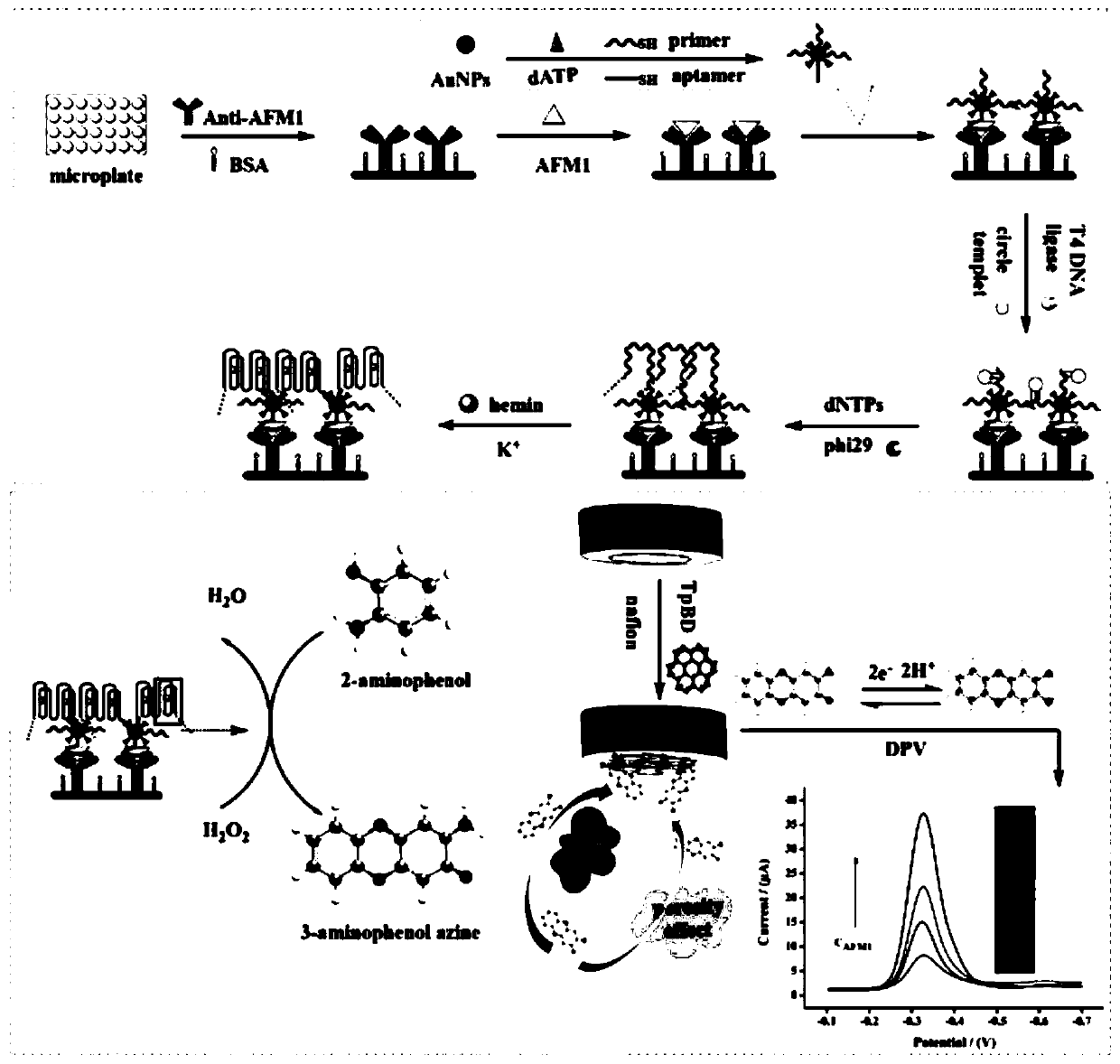
[0054] Example 1 Electrochemical-ELISA Immunosensor Construction
[0055] (1) Primer-AuNPs-aptamer complex preparation:
[0056] Au-S bonds formed between AuNPs and sulfhydryl-modified nucleic acid chains to prepare primer-AuNPs-aptamer complexes: mix 1 mL of prepared AuNPs (7.5 μM) with 3 μL sulfhydryl-modified aflatoxin M1 aptamers (0.1 mM), 12 μL of sulfhydryl-modified primers (0.1 mM) and 2 μL of acetic acid (0.5 mM, pH 5.2) were mixed and incubated at room temperature for 12 h; then 20 μL of dNTP (0.1 mM) was added to block redundant active sites on the surface of AuNPs. Subsequently, 1 mL of NaCl (0.25M) was added and incubated at room temperature for 24 h to increase the stability of the complex. Excess reagents were removed by centrifugation at 10000rpm for 20min; the resulting precipitate was suspended in 1.5mL Tris-EDTA (TE, 0.1M, pH 7.4) buffer, and stored at 4°C for later use;
[0057] (2) Construction of primer-AuNPs-aptamer / aflatoxin M1 / antibody sandwich modifi...
Embodiment 2
[0079] Example 2 Constructing the standard curve of the target antigen aflatoxin M1
[0080] The standard solution is processed with reference to the sensor in Example 1:
[0081] Replace the aflatoxin M1 target solution in step (2) with different concentrations of aflatoxin M1 standard solutions (0.1, 0.25, 0.5, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 100, 120ng / mL), respectively, to obtain the ELISA plates modified by the primer-AuNPs-aptamer / aflatoxin M1 / antibody sandwich structure containing different concentrations of aflatoxin M1;
[0082] Then measure the corresponding reduction peak current value I according to (3), (4) steps respectively i , Measure the reduction peak current value I of blank sample (do not contain aflatoxin M1) simultaneously 0 , using I i / I 0 Construct a linear model with concentrations, such as Figure 7 shown in I i / I 0 is the ordinate, the concentration is the abscissa, the standard curve is y=2.076+0.039x, the correlation coefficient R 2...
Embodiment 3
[0084] Embodiment 3 sensor construction condition optimization experiment
[0085] (1) Effect of primer / aptamer ratio:
[0086] Primer / aptamer ratios were investigated with varying primer / aptamer ratios (2:1, 3:1, 4:1, 5:1, 6:1, and 7:1, with a fixed aptamer concentration of 1.5 μM) Impact on analysis performance.
[0087] Such as Figure 8 A, As the primer / aptamer ratio increases, the current signal increases, and when the primer / aptamer ratio is higher than 4:1, the current signal decreases. The reduction in current may be due to the extra primers occupying the binding sites of AuNPs that belong to aptamers, and may even lead to no aptamers in the complex. Therefore, primer-AuNPs-aptamer complexes were synthesized using a primer / aptamer ratio of 4:1.
[0088] (2) Optimization of rolling circle amplification reaction time:
[0089] The concentration of T4 DNA ligase and phi29 polymerase has a great influence on the rolling circle amplification reaction. Such as Figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com