Preparation method and application of carbendazim hapten and antigen
A carbendazim and hapten technology, applied in the field of preparation of carbendazim haptens and antigens, can solve the problems of inability to perform large-scale on-site detection, poor timeliness, and cumbersome processing, and achieve rapid detection, good affinity, and crossover. The effect of low response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of carbendazim hapten
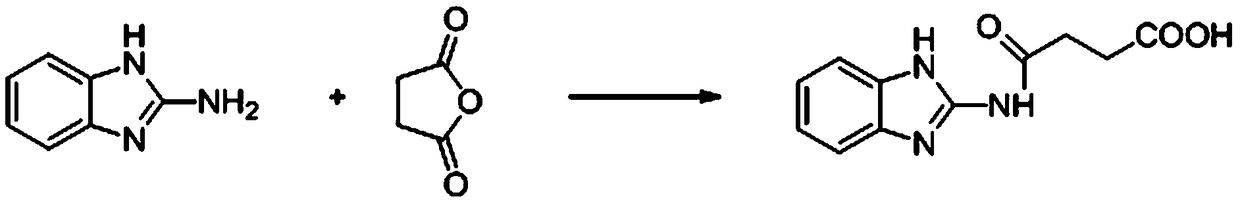
[0025] 1. Synthesis of carbendazim hapten (see the synthetic route figure 1 )
[0026] Dissolve 1.05 g (10.5 mmol) of succinic anhydride in 100 mL of acetonitrile, add 1.33 g (10 mmol) of 2-aminobenzimidazole in portions under heating and reflux, continue to reflux for 1 hour, cool to room temperature, filter to obtain a solid, and , washed with acetonitrile and dried to obtain 2.00 g of carbendazim hapten with a yield of 86%.
[0027] 2. Identification of carbendazim hapten
[0028] 1H NMR (DMSO-d6): 11.95 (br, 3H), 7.43 (d,d, J1 = 5.53 Hz, J2 =5.61 Hz), 7.07 (d,d, J1 = 5.53 Hz, J2 = 5.61 Hz) , 2.70 (d, J = 6.61 Hz, 2H), 2.58 (d, J = 6.61 Hz, 2H).
[0029] In the spectrum, chemical shifts δ=2.70 and 2.58 are resonance absorption peaks of methylene hydrogen on the spacer arm. The existence of these peaks proves that the spacer arm coupling is successful and the structure of the carbendazim hapten is correct.
Embodiment 2
[0030] Example 2 Preparation of carbendazim antigen
[0031] 1. Synthesis of carbendazim immunogen
[0032] The carbendazim hapten was conjugated with bovine serum albumin (BSA) to obtain the immunogen.
[0033] Take 15 mg of carbendazim hapten, dissolve it in 1 mL of N,N-dimethylformamide (DMF), add 0.22 mL of oxalyl chloride, and stir overnight to obtain hapten activation solution A; take bovine serum albumin ( BSA) 50 mg, add 3.6 mL of 0.05 M borate buffer solution to dissolve to obtain liquid B, add liquid A dropwise to liquid B, stir at 4°C for 5 h, stop the reaction, dialyze with 0.01 mol / L PBS at 4°C for 3 d. Change the dialysate 3 times a day. Aliquoted to obtain the carbendazim-BSA antigen and stored at -20°C for future use.
[0034] 2. Synthesis of carbendazim coating agent
[0035] The carbendazim hapten was coupled with ovalbumin (OVA) to obtain the coating source.
[0036] Take 13 mg of carbendazim hapten, dissolve it in 1 mL of DMF, add 0.21 mL of isopropyl ...
Embodiment 3
[0045] Example 3 Preparation of carbendazim monoclonal antibody
[0046] 1. Obtaining hybridoma cells
[0047] 1) The first immunization: fully emulsify the carbendazim hapten-BSA conjugate (immunogen) with 3 times Freund's complete adjuvant, and subcutaneously inject Balb / c mice aged 8-10 weeks at an immunization dose of 150 μg / piece;
[0048] 2) Booster immunization twice: from the first immunization, booster immunization once every two weeks, with Freund's incomplete adjuvant instead of Freund's complete adjuvant, the method and dosage are the same as the first immunization;
[0049] 3) One week after the last booster immunization, the fundus vein blood was collected to measure the titer and inhibition. When there was inhibition and the titer reached more than 1:10000, the following last immunization was carried out: intraperitoneal injection of 0.1 mL of the immunogen solution without any adjuvant, and then executed three days later Mice, whose spleen was fused with myel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com