Flow system and methods for digital counting
a flow system and digital counting technology, applied in fluid controllers, laboratory glassware, instruments, etc., can solve the problems of inability to carry out digital techniques, analysis ends, and digital techniques can be relatively complex, and achieve the effect of reducing the evaporation of each nano-to-attoliter dropl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formation and Preservation of a Femtoliter Aqueous Microdroplet Array
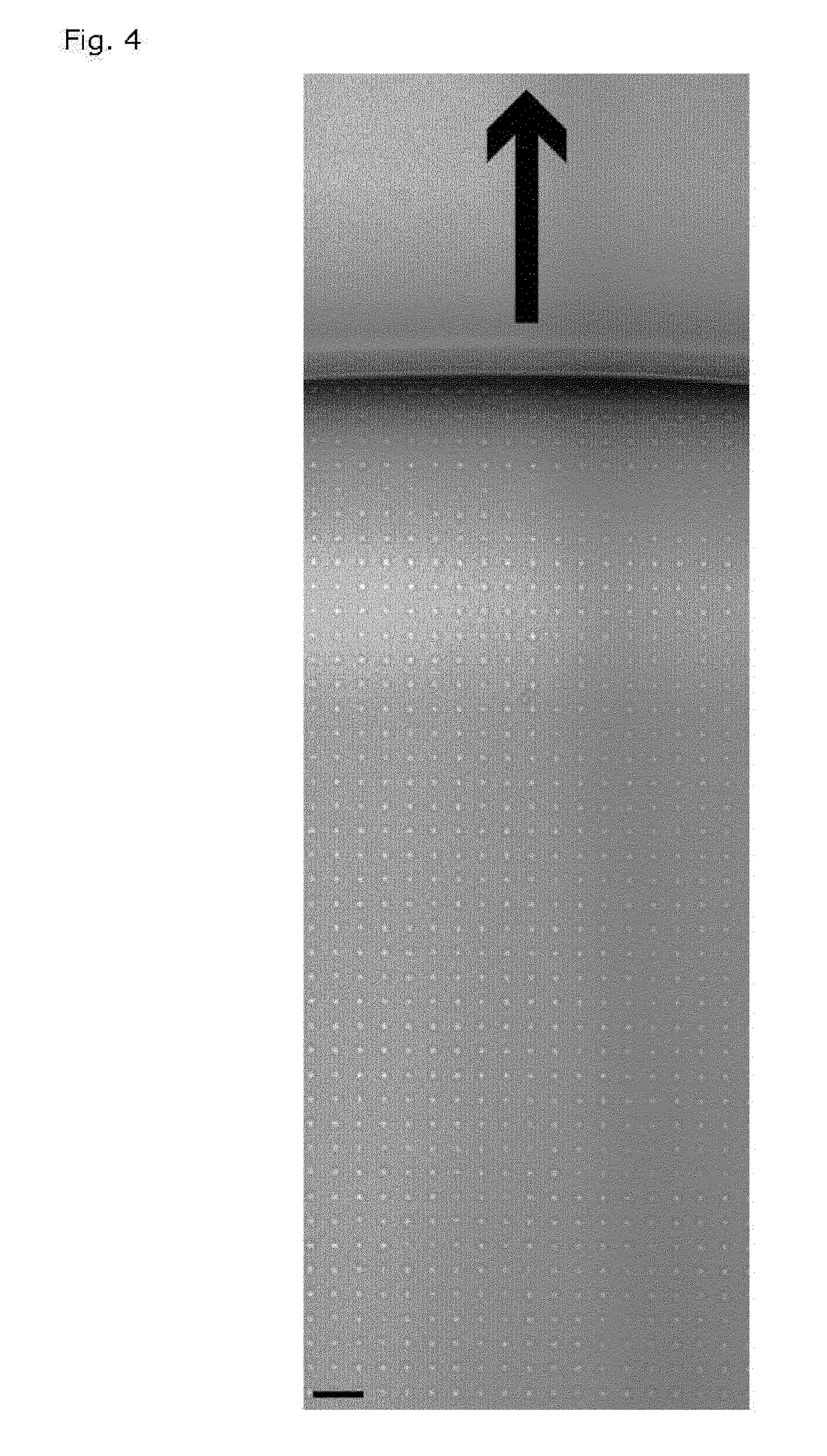
[0320]To form stable microdroplets, a regular quadratic array of hydrophilic circular features embedded on a planar hydrophobic region was contacted with a phosphate buffered aqueous solution. A 10 μl plug of the solution was actuated across the surface of the array, thus leaving microdroplets behind on the hydrophilic features as shown on the micrograph in FIG. 4.
[0321]The flow system was defined by two openings at each end of a rectangular channel to guide the liquid. The width of the channel was 3 mm, the length was 16 mm and the height was 150 μm. The array was placed centrally in the channel, with a width of 2.9 mm, a length of 14 mm and comprised a total of 406,000 hydrophilic features. The diameter of the hydrophilic circles was 5 μm, and the intercircle spacing was 10 μm. The contact angle of the aqueous solution on the hydrophobic surface was approx. 110 degrees and the experiment was conducted at ambient ...
example 2
How to Render an Array of Aqueous Micro-Droplets Evaporation-Resistant by Optimizing Flowchannel-, Droplet- and Array-Geometry
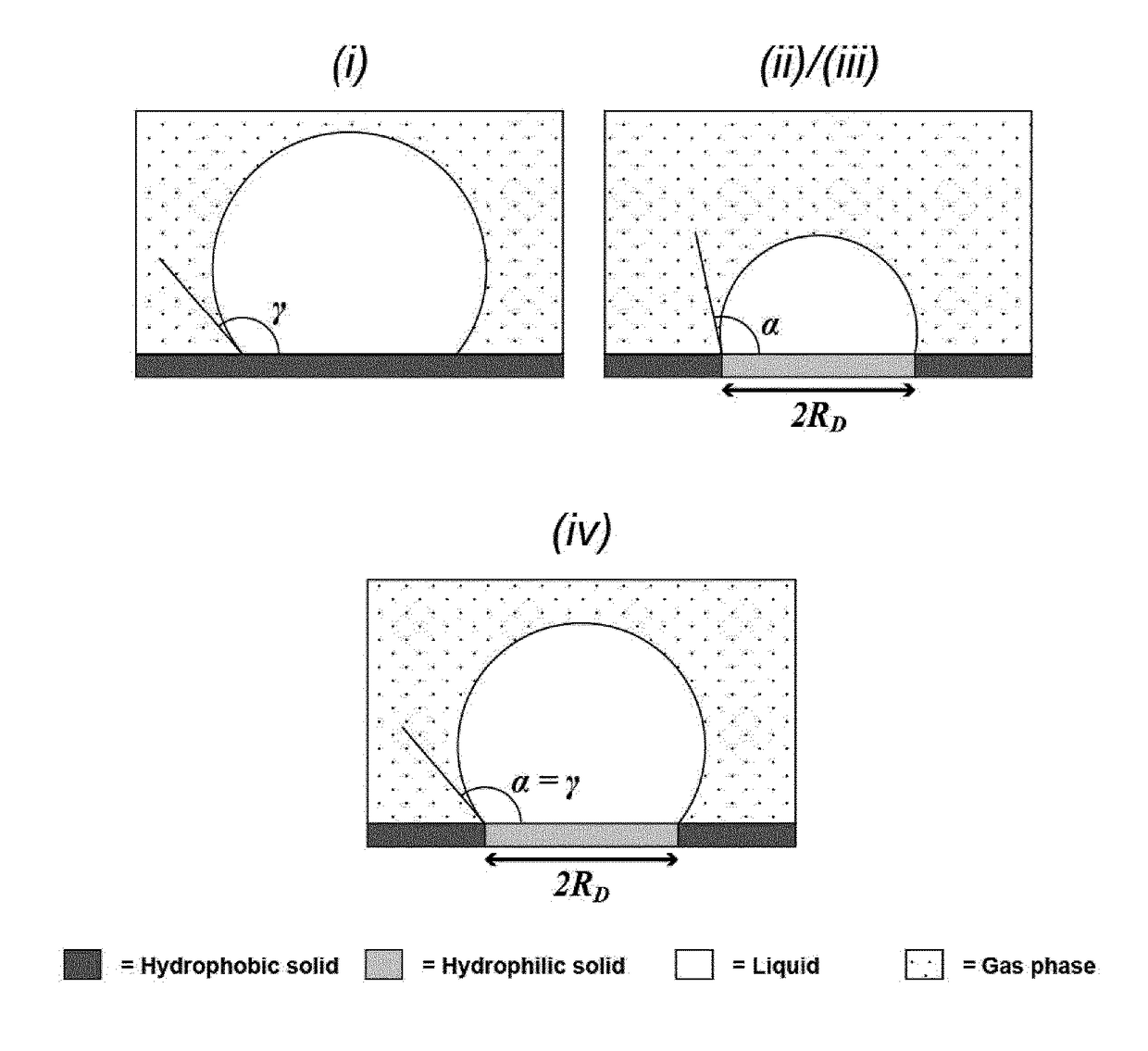
[0323]Consider a flow channel in which a chemically patterned solid substrate has been embedded. The chemical pattern consists of circular hydrophilic regions organized into an array. The hydrophilic array is surrounded by a continuous hydrophobic region. In this way, an array of microdroplets is formed on top of the hydrophilic features once an aqueous solution is infused and subsequently withdrawn from the flowchannel, as illustrated in Example 1.
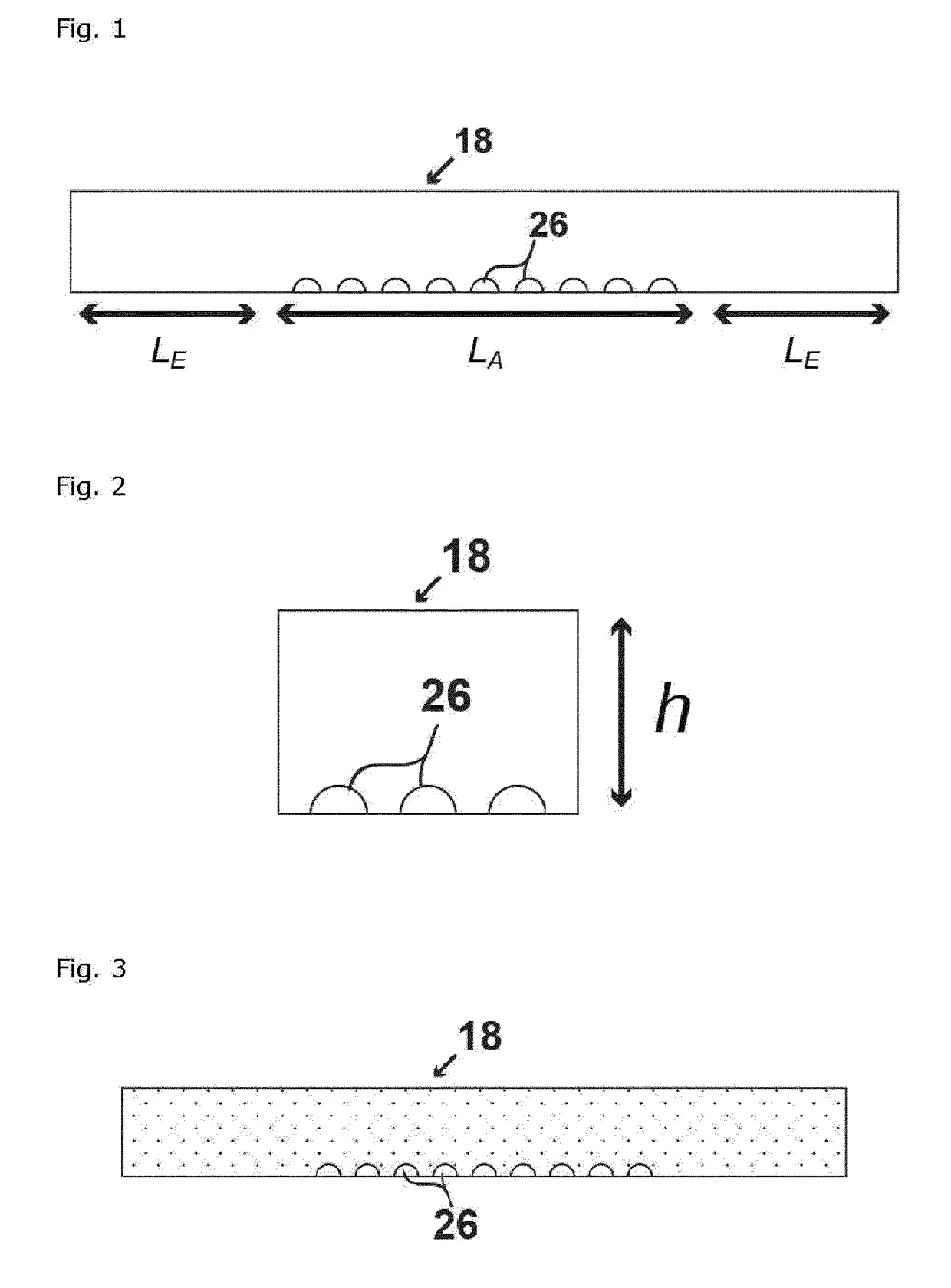
[0324]The dimensions of the flow channel are defined on FIG. 13, and are characterized by h, which is the height of the channel, lA, which is the length of the flowchannel covered by the array, lE, which is the length of the excess part of the channel leading to the inlet / outlet, but not hosting the array. The parameters defining the array are the droplet radius RD, defined as the radius of the hydrophilic feature ...
example 3
Fabrication of a Flow System
[0333]Fabrication of a flow system took place in two main steps; one step utilizes UV photolithography and microfabrication processing to produce the patterned hydrophilic features, whereas the second step deals with integrating the hydrophilic pattern into a flow compartment exhibiting the right geometry. Below both steps will be described in more detail.
[0334]Microfabrication of a Patterned Hydrophilic Substrate.
[0335]In this embodiment of the invention, the hydrophilic features were composed of quartz (SiO2) and the hydrophobic region was composed of perfluorodecyltrichlorosilane (FDTS). In the first step of the fabrication process, a molecular monolayer of FDTS was deposited on the quartz wafer by molecular vapor deposition using an MVD 100 Molecular Vapor Deposition system (Applied Microstructures Inc.). The FDTS underwent covalent attachment to silanol groups on the surface of the quartz and hence produced a hydrophobic monolayer on the wafer surfac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| droplet radius | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com