Preparation method and application of a kind of coumarin hapten and antigen
A coumarin and hapten technology applied in the field of immunochemistry to achieve the effects of high antibody specificity, rapid detection, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of Coumarin Hapten
[0021] 1. Synthesis of coumarin hapten
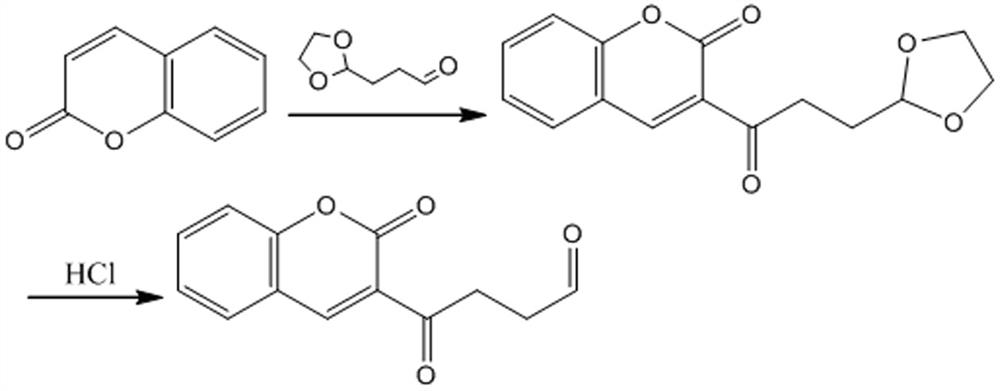
[0022] Take 1.00 g of coumarin, add 80 mL of chlorobenzene and stir to dissolve, add 2.50 g of tert-butanol hydroperoxide, stir and mix well, add 3-(1,3-dioxa-2-yl) propanal 0.89 g, stirred at 100°C for 24 h. After stopping the reaction, add 100 mL of cold water, extract 3 times with 100 mL of ethyl acetate, wash with 50 mL of water, dry the organic phase over anhydrous sodium sulfate and evaporate to dryness, and 60 mL of petroleum ether / dichloromethane (v / v, 1 / 1 ) recrystallized to obtain 1.70 g of acetal coumarin compound with a yield of 94.00%.
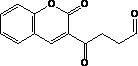
[0023] Take 1.70 g of acetal coumarin, add 20 mL of acetonitrile to dissolve, add 10 mL of 1 mol / L dilute hydrochloric acid, stir vigorously at room temperature for 4 hours, stop the reaction, rotary evaporate, remove acetonitrile, add 50 mL of water, add 1 mol / L NaOH to adjust When the pH value reaches 6, add 70 mL of 1,2-dichloroethane ...
Embodiment 2
[0027] Example 2 Preparation of Coumarin Antigen
[0028] 1. Synthesis of coumarin immune antigen
[0029] The coumarin hapten is conjugated with bovine serum albumin (BSA) to obtain the immune antigen.
[0030] Take 16 mg of coumarin hapten, add ethanol to dissolve to obtain liquid A, take 0.1 g of bovine serum albumin, add acetate buffer solution with a pH value of 5.6 to dissolve to obtain liquid B, add liquid A to liquid B dropwise Stir at 4°C for 20 h, add 1 mg sodium borohydride, continue to stir for 2 h, dialyze and purify with 0.02 mol / L PB for three days, change the medium three times a day, aliquot the immunogen, and store it at -20°C for future use.
[0031] 2. Synthesis of coumarin-coated antigen
[0032] The coumarin hapten was conjugated with ovalbumin (OVA) to obtain the coating antigen.
[0033] Take 5 mg of coumarin hapten, add ethanol to dissolve to obtain liquid A, take 50 mg of ovalbumin, add carbonate buffer solution with a pH value of 9.1 to dissolve to ...
Embodiment 3
[0036] Example 3 Preparation of coumarin monoclonal antibody
[0037] 1. Obtaining hybridoma cells
[0038] 1) Animal immunization: Fully emulsify the coumarin hapten-BSA conjugate (immunogen) with an equal amount of Freund's complete adjuvant, and inject 6-week-old Balb / c mice subcutaneously, 0.2 mL each; thereafter , a booster immunization every two weeks, with Freund's incomplete adjuvant instead of Freund's complete adjuvant, the method and dosage are the same as the first immunization;
[0039] 2) One week after the last booster immunization, the fundus vein blood was collected to measure the titer and inhibitory effect of coumarin antibody. When there was inhibition and the titer reached 1:10000 or more, the following final immunization was performed: intraperitoneal injection of immunogen solution without any adjuvant 0.1 mL, the mice were sacrificed three days later, and the spleen lymphatic B cells were fused with myeloma cells;
[0040] 3) After 7 days of cell fusi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com