Methyl parathion hapten, and preparation method and application thereof
A methyl parathion, hapten technology, applied in chemical instruments and methods, fibrinogen, animal/human proteins, etc. The effect of low cost, good affinity and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
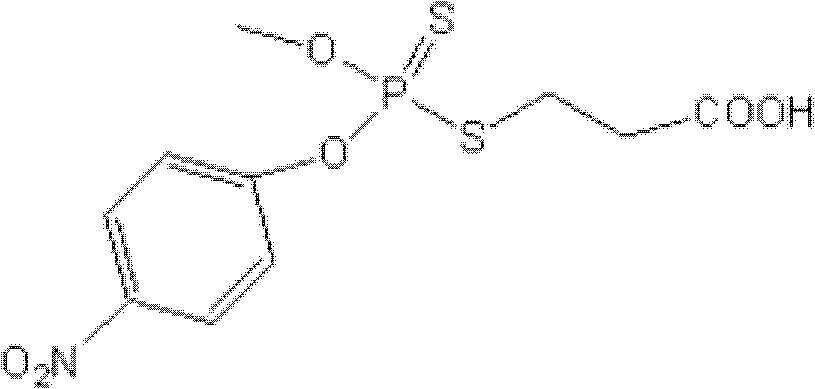
[0020] Synthesis and identification of embodiment 1 methyl parathion hapten
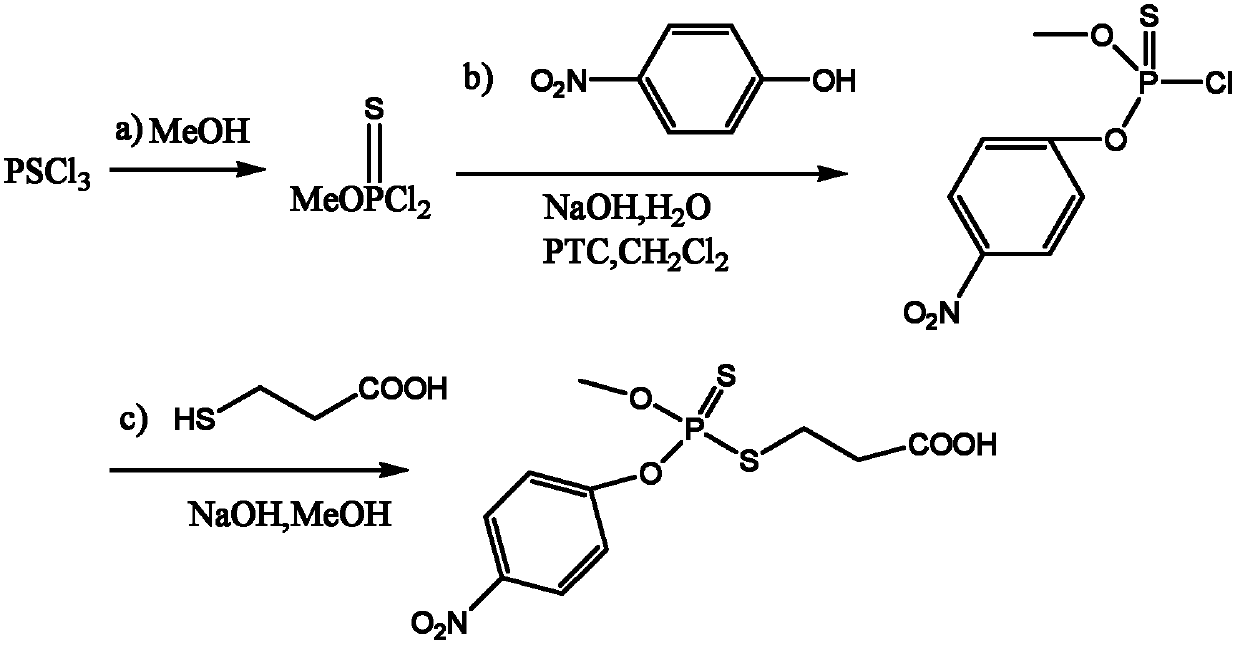
[0021] One, the synthesis of methyl parathion hapten (synthetic route such as figure 1 )
[0022] a) Stir the phosphorous trichloride into a single-necked bottle, slowly add anhydrous methanol (1:4 molar ratio to phosphorous trichloride) dropwise, keep the temperature at 10°C, continue the reaction for 1 hour after dropping, wash with a large amount of cold water, Static layering, the lower layer is the target object, dried with anhydrous magnesium sulfate;
[0023] b) Dissolve p-nitrophenol and sodium hydroxide (molar ratio 1 / 3) in (water / dichloromethane, 1 / 3, v / v), add PTC, stir for about 20min, slowly drop it into the solution containing O - in a single-necked bottle of methylphosphoryl dichloride (molar ratio to p-nitrophenol 1 / 2), keep the temperature at 10°C, react for 5h, evaporate the reaction solution to dryness, and purify by column chromatography (ethyl acetate / petroleum ether, 1 / 20, v / ...
Embodiment 2
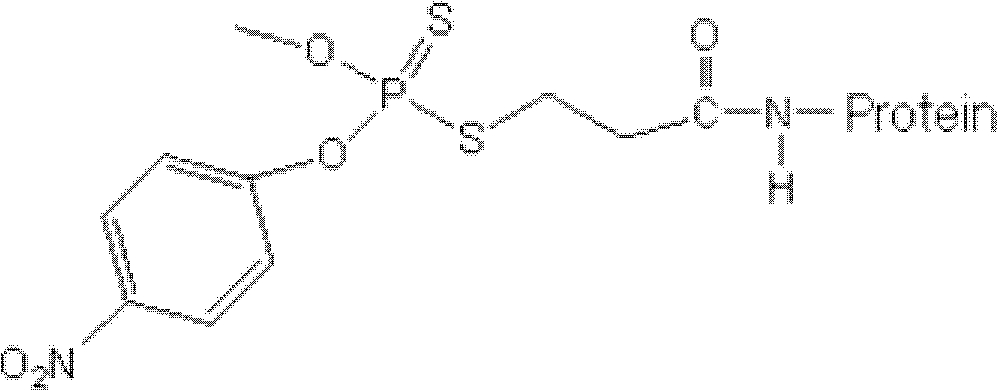
[0027] Example 2 Methyl parathion antigen
[0028] The methyl parathion hapten is coupled with the carrier protein to obtain the methyl parathion antigen.
[0029] 1. Preparation of immunogen - synthesis of methyl parathion hapten-bovine serum albumin conjugate
[0030] Fully dissolve 30 mg of methyl parathion hapten in 1 ml of N,N-dimethylformamide (DMF) to obtain solution I; weigh 50 mg of bovine serum albumin (BSA), and dissolve it in 2 ml of PBS ( pH 7.2), slowly add solution I to the BSA solution drop by drop to obtain solution II; weigh 12.5mg of carbodiimide (EDC) and dissolve it in 1ml of water, slowly add it to solution II at room temperature, and stir for 24h ; Dialyze with 0.01mol / L PBS for 3 days, change the dialysate twice a day to remove unreacted small molecular substances; centrifuge at 12000r / min for 30min, collect the supernatant to obtain the methyl parathion immunogen, aliquot, Store at -20°C for later use.
[0031] 2. Preparation of coating agent—synthe...
Embodiment 3
[0035] Embodiment 3 methyl parathion monoclonal antibody
[0036] 1. Preparation of methyl parathion monoclonal antibody
[0037] Animal immunization: Inject the immunogen into the body of Balb / c mice with an immunization dose of 150 μg / mouse to make it produce polyclonal antibody serum.
[0038] Cell fusion and cloning: After the measurement result of mouse serum was higher, the splenocytes were taken and fused with SP2 / 0 myeloma cells at a ratio of 8:1, and the cell supernatant was measured by indirect competitive ELISA, and the positive wells were screened. Positive wells were cloned by limiting dilution until hybridoma cell lines secreting monoclonal antibodies were obtained.
[0039] Cell cryopreservation and recovery: the monoclonal hybridoma cell line of methyl parathion was made into 1×10 9 cells / ml for long-term storage in liquid nitrogen. When recovering, take out the cryopreservation tube, put it into a 37°C water bath to thaw quickly, remove the cryopreservation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com