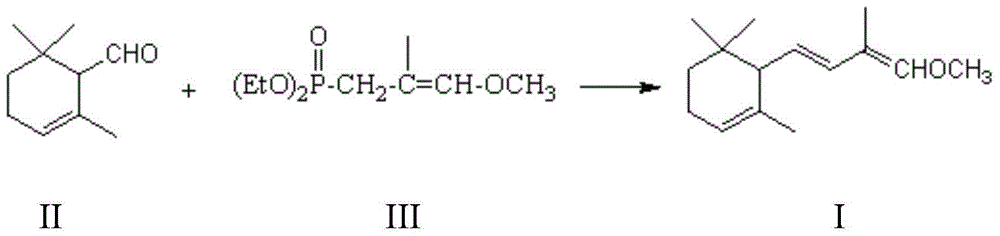1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-1,3-butadiene and preparation method thereof
A technology of cyclohexene and methoxy, which is applied in the field of intermediate 1-methoxy-2-methyl-4--1, can solve the problems of poor atom economy, achieve low cost, easy to obtain raw materials, and advanced technology The effect of simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
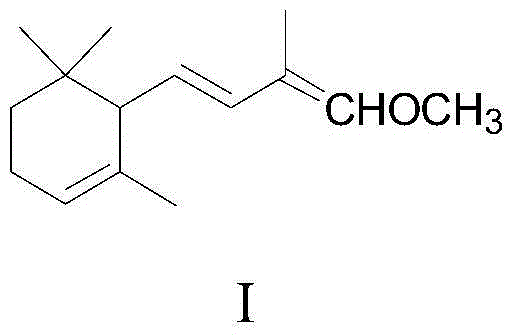
[0030] Example 1: Preparation of 1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-1,3-butadiene
[0031] Add potassium tert-butoxide (12.3g, 0.11mol) and a mixture of tetrahydrofuran and dimethyl sulfoxide (50mL, 8:1, V / V) into a 250mL three-neck flask protected by nitrogen, keep it warm in a cold bath, and stir mechanically Then, add the phosphonate (24.4g, 0.11mol) shown in formula III dropwise, keep at -20°C for about half an hour to complete the dropwise addition, continue to keep warm and stir for about 1 hour to make the carbanion dissociation reaction fully; then keep at -20°C Add α-cyclocitral (15.2g, 0.1mol) represented by formula II dropwise, and the dropwise addition is completed in about 1 hour. Continue to keep warm and stir for about half an hour. After the reaction is completed by gas chromatography, add water (50mL) and diethyl ether (100mL) Stir for 10 minutes, separate layers, wash the ether layer with 5% aqueous sodium chloride solution (25mL*3), dry...
Embodiment 2~8
[0037] Examples 2-8: Condensation reactions under different bases, solvents and reaction temperatures to prepare 1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene- 1-yl)-1,3-butadiene
[0038] Add a certain amount of alkali and 20mL of a certain solvent into a 250ml three-necked flask protected by nitrogen gas (see Table 1 for the types of alkali and solvent), keep it warm in a cold bath, and add a certain amount of phosphine shown in formula III dropwise under mechanical stirring. 20mL of the above-mentioned solvent of the acid ester (see Table 1 for the molar amount), kept at a certain temperature for about half an hour to complete the dropwise addition, and continued to insulate and stir for about 1 hour to make the carbanion dissociation reaction fully; then keep the above temperature, dropwise added The above solvent of α-cyclic citral (7.6g, 0.05mol) was added dropwise in about 1 hour, and continued to keep stirring at the above temperature for about half an hour. Afte...
Embodiment 9
[0049] Example 9: Preparation of 2-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butene-1-aldehyde
[0050] Add the 1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl) prepared in Example 1 to the 250mL three-necked flask protected by nitrogen -1,3-butadiene (11.0g, 0.05mol), tetrahydrofuran (100g) and p-toluenesulfonic acid (0.7g), stir well, add water (22g) dropwise, and stir at 20-25°C for 24 hours, Gas chromatographic tracking reaction is basically complete, add a solution configured by 2g sodium bicarbonate and 20g water to neutralize, steam the tetrahydrofuran under reduced pressure with a water pump, then add 100mL cyclohexane, separate layers, and the organic layer is washed with 30mL water After drying with magnesium sulfate over water, the solvent was recovered under reduced pressure to obtain the crude product of 2-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butene-1-aldehyde 10.1g, GC content 73.1%, yield is 71.7%.
[0051] The structure of the product i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com