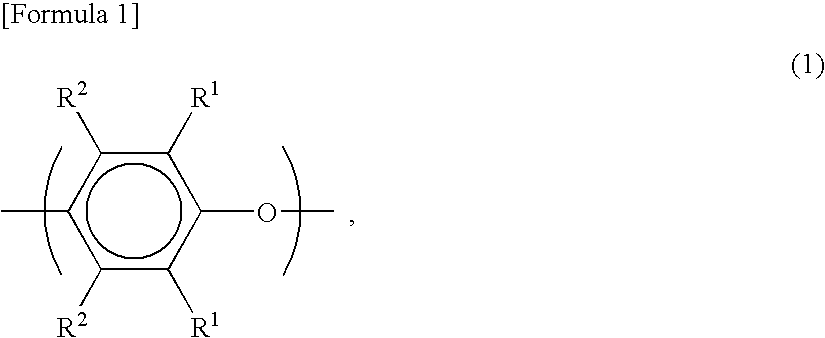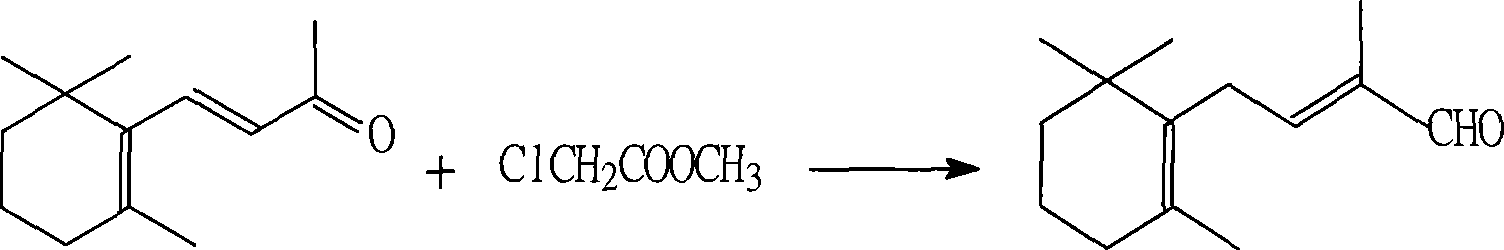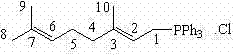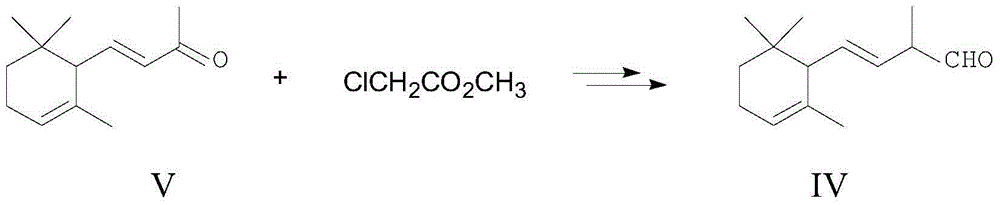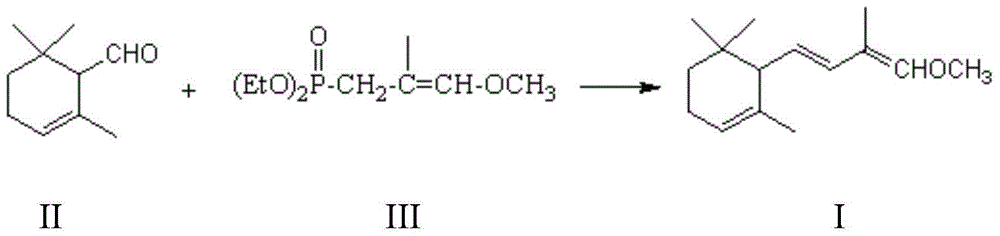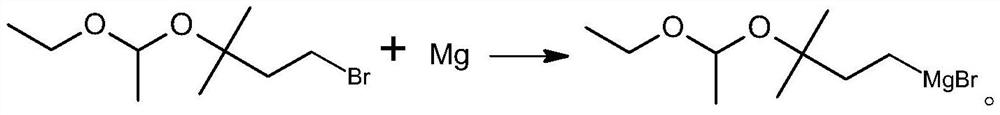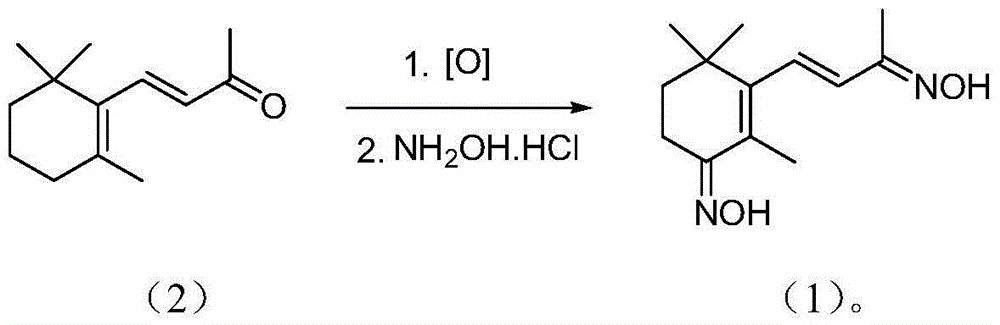Patents
Literature
41results about How to "Industrial value" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fiber-reinforced thermoplastic resin molded article
ActiveUS7858172B2Improve accuracyGood lookingLamination ancillary operationsSynthetic resin layered productsHeat resistanceUltimate tensile strength
Provided is a fiber-reinforced thermoplastic resin molded article, which contains reinforcing fibers having a given flattened cross-sectional profile, in which the fiber length distribution of the reinforcing fibers is shifted on the side of long fibers, and which is excellent in mechanical strength, heat resistance, dimensional accuracy such as warpage resistance, and surface appearance.
Owner:MITSUBISHI ENG PLASTICS CORP
Fiber-reinforced thermoplastic resin molded article
ActiveUS20100009158A1High dimensional accuracyImprovement of outward appearanceSynthetic resin layered productsCeramic shaping apparatusPolymer sciencePolymer chemistry
Provided is a fiber-reinforced thermoplastic resin molded article, which contains reinforcing fibers having a given flattened cross-sectional profile, in which the fiber length distribution of the reinforcing fibers is shifted on the side of long fibers, and which is excellent in mechanical strength, heat resistance, dimensional accuracy such as warpage resistance, and surface appearance.A fiber-reinforced thermoplastic resin molded article of a thermoplastic resin composition comprising from 70 to 35% by weight of a thermoplastic resin (A), and from 30 to 65% by weight of reinforcing fibers (B) of which the cross section is flattened to have a degree of flatness, as expressed by the formula mentioned below, of at least 2.3, wherein the weight-average fiber length of the reinforcing fibers in the molded article is at least 1 mm:Degree of flatness=major diameter of reinforcing fiber (a) / minor diameter of reinforcing fiber (b).
Owner:MITSUBISHI ENG PLASTICS CORP
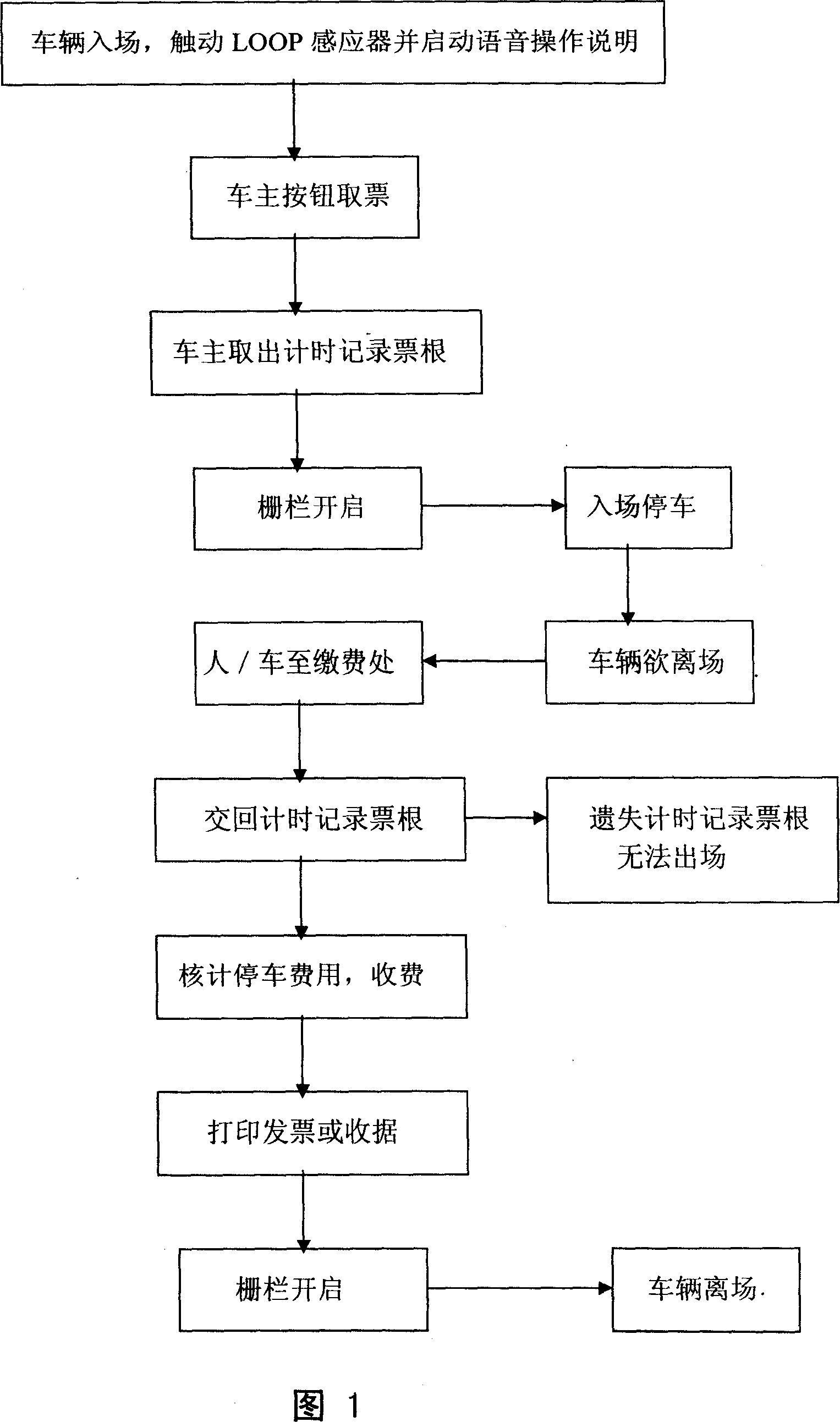
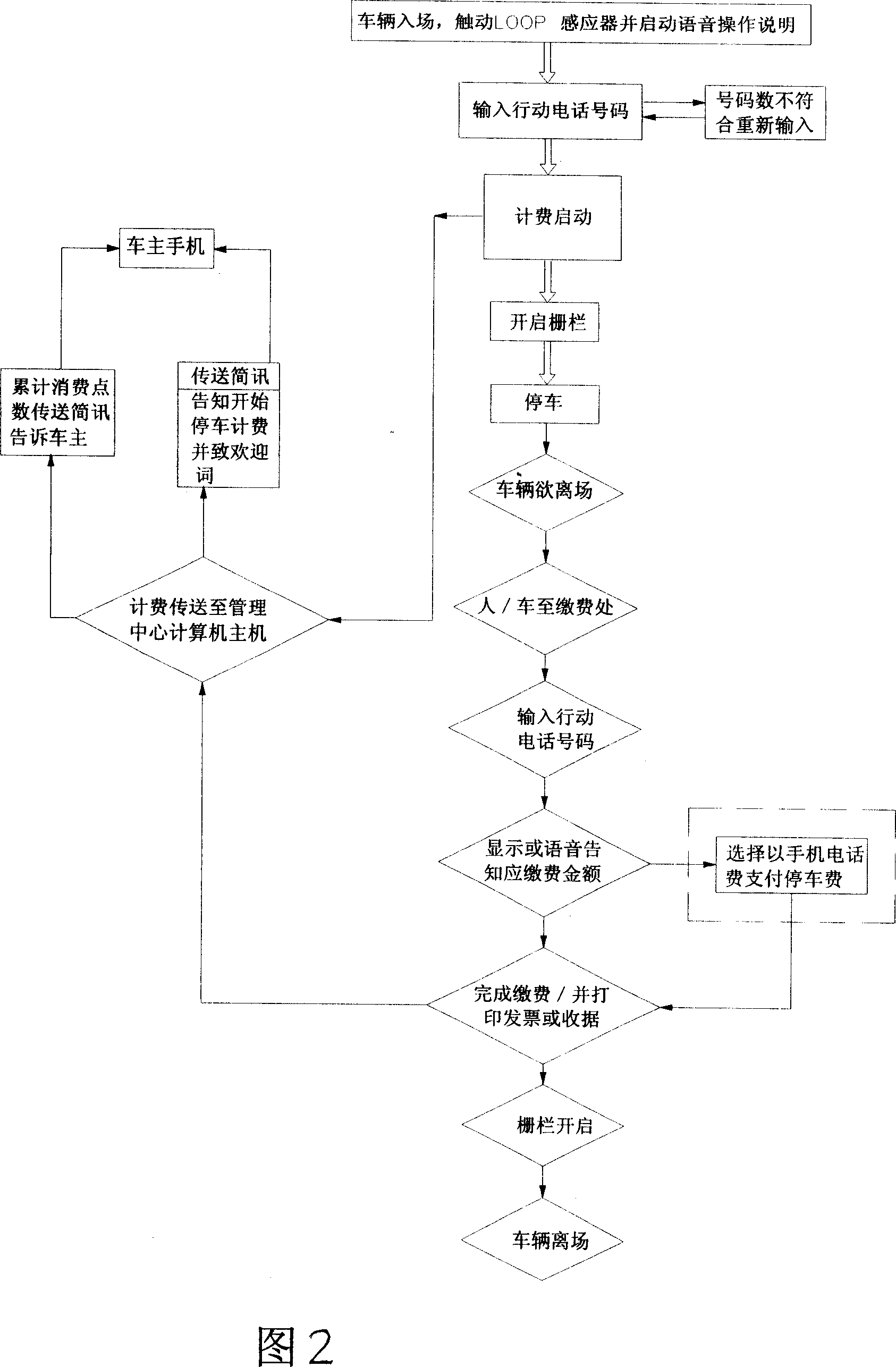
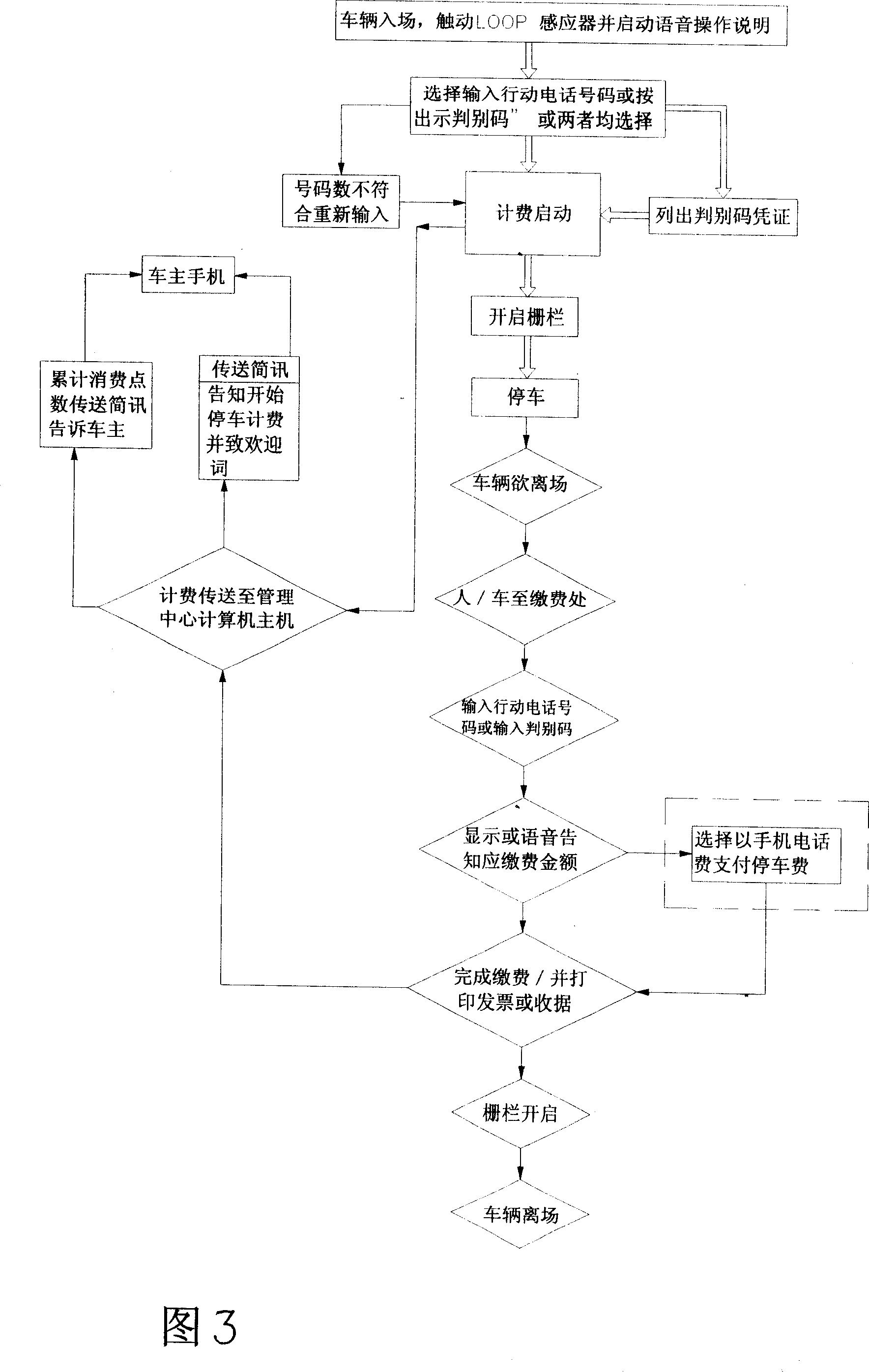
Parking lot fee collection management system
InactiveCN101071519AImprove accounting work efficiencyRight to self-determinationApparatus for meter-controlled dispensingPaymentCollection management
A parking fees management system, mainly by vehicle sensors admission touchs LOOP voice activated instructions from the owners of their own actions importation owners choose telephone numbers and billing methods, in order to open the fence to enter Parking, admission to leave to pay, the owner of the action again importation of telephone numbers, so that the management system to show or voice should inform the parking fee and the amount of time by the choice of site owners pay their actions or merger of telephone numbers in the invoice payment methods, completed Payment and print receipts or invoices, will open the fence, vehicles left in the effective elimination of open car parking vouchers from stealing cars and to prevent the loss of safety management effectiveness, but also provide owners with a mobile phone number as a means of importation convenience, and the effective control of upgrading the car park and operational efficiency.
Owner:颜振
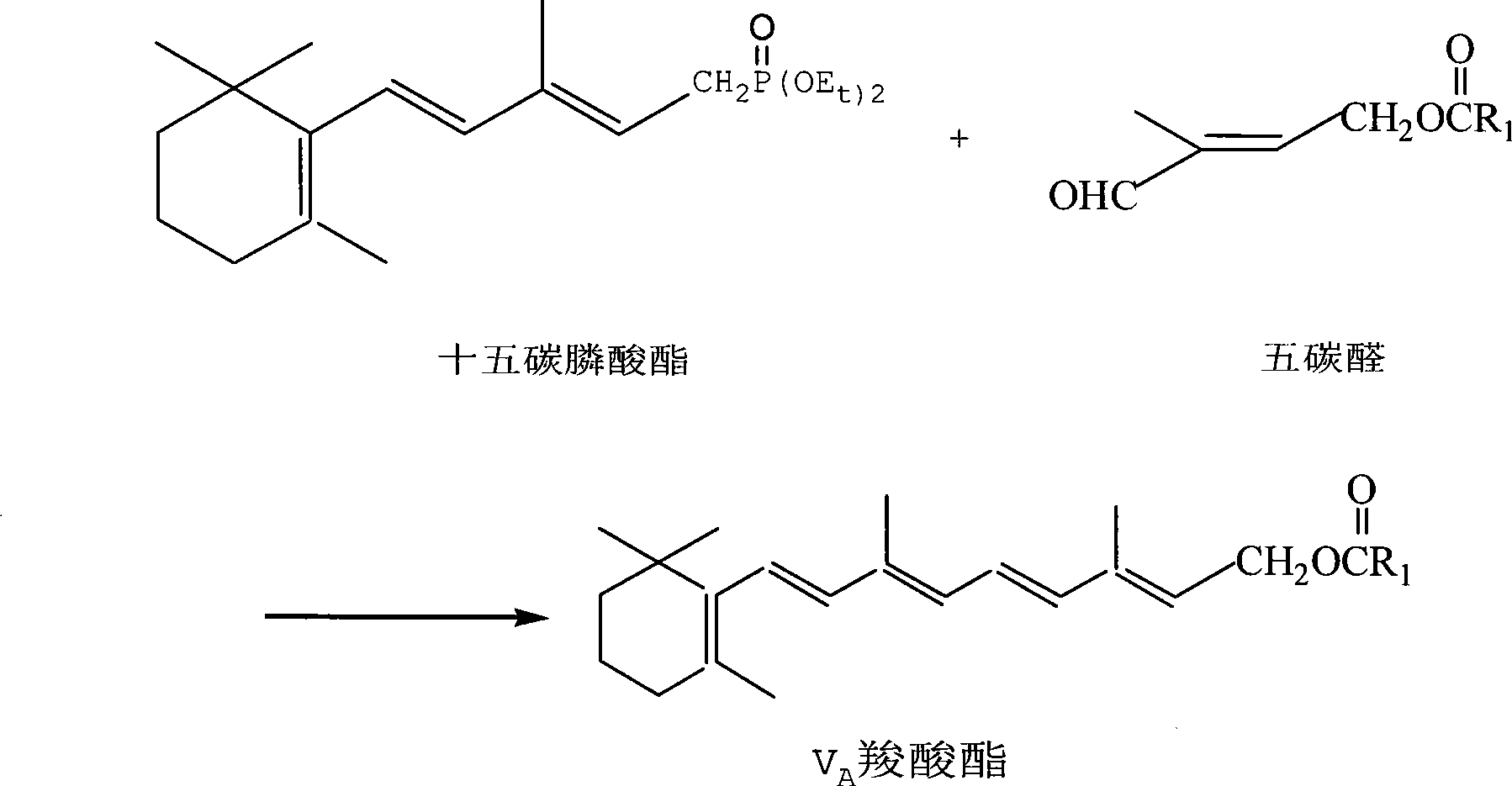
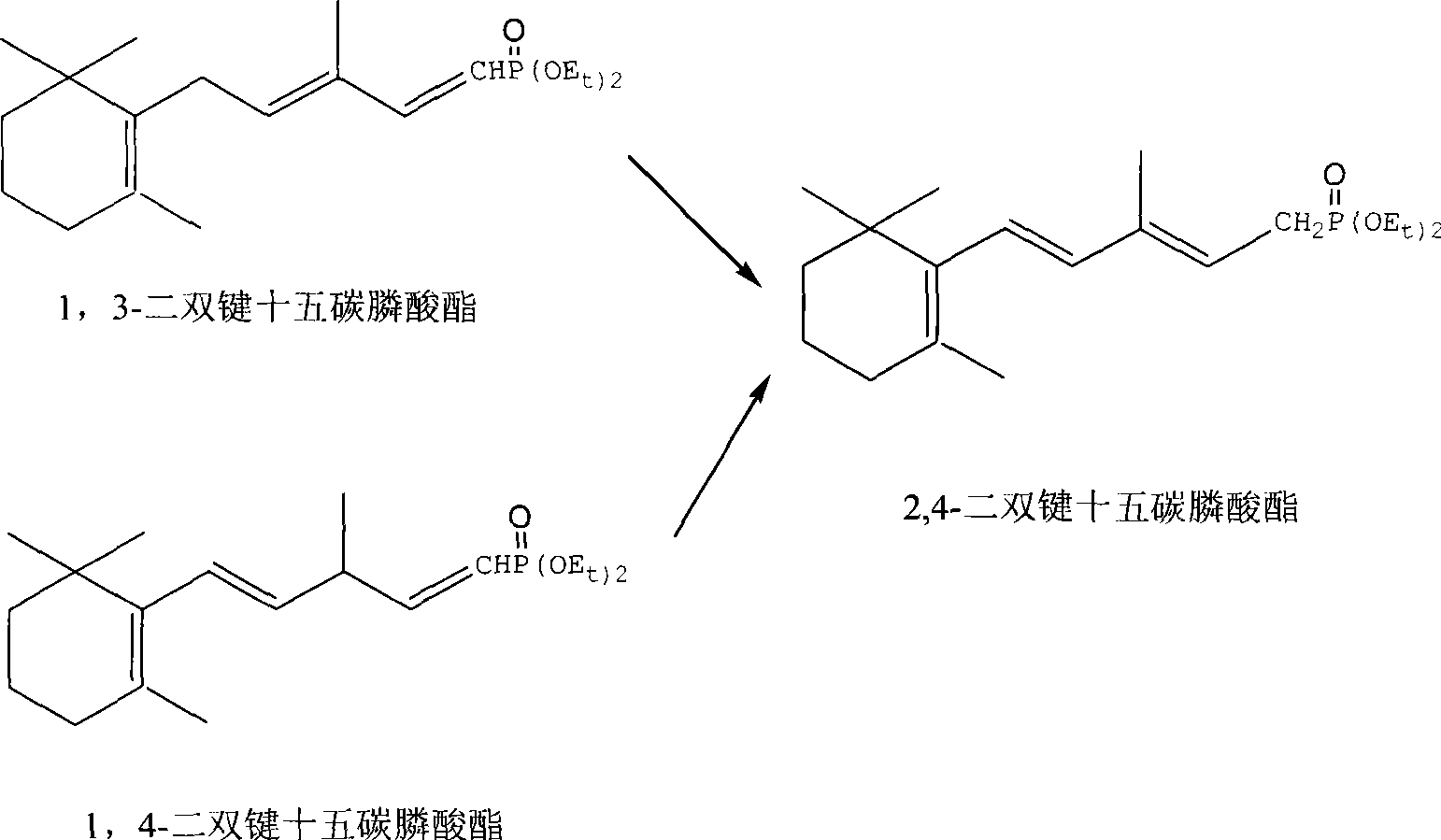
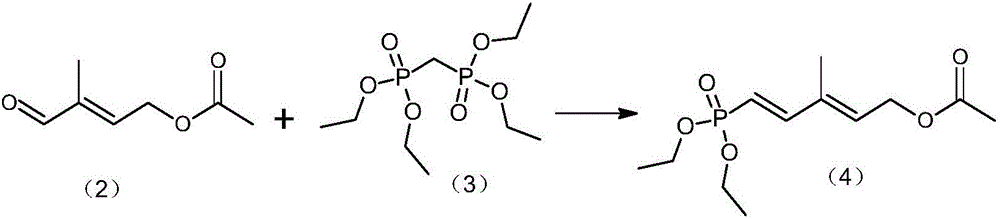
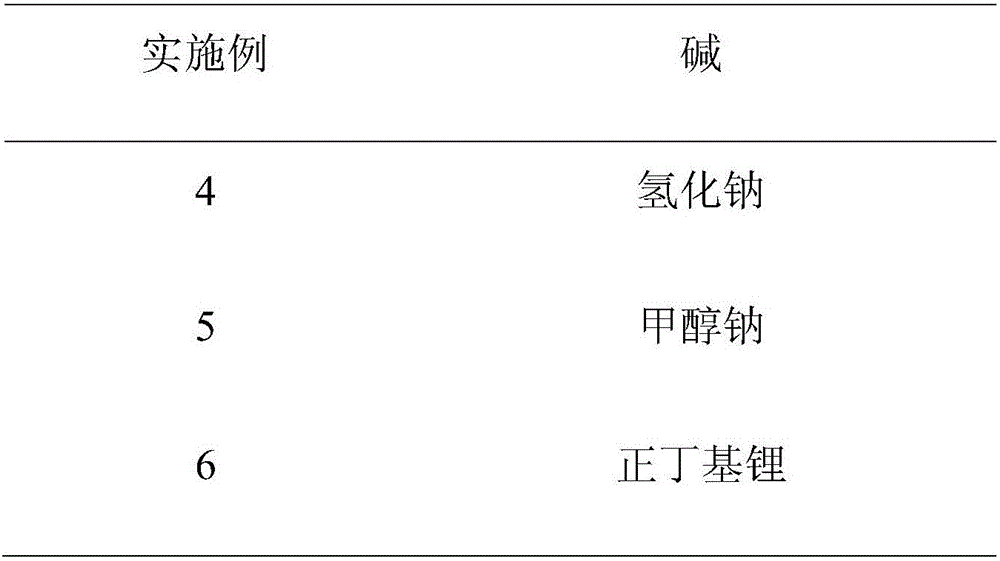
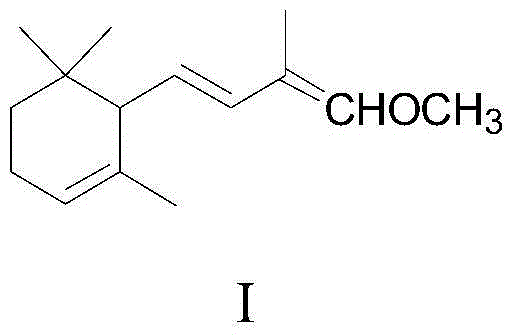
Method for preparing 2,4-di-double-bond 15-carbon phosphonate
InactiveCN101544668ASimple process routeIndustrial valueGroup 5/15 element organic compoundsEthyl esterDouble bond
The invention discloses a method for preparing a vitamin A intermediate 2,4-di-double-bond 15-carbon phosphonate. The prior methods use beta-ionone as a raw material and have long synthetic routes which are often more than three steps. The method n uses the beta-ionone as the raw material to perform condensation reaction with ethylene tetraethyl diphosphonate to obtain the target compound 2,4-di-double-bond 15-carbon diethyl phosphonate. The method uses the beta-ionone as the raw material to generate the target product 2,4-di-double-bond 15-carbon phosphonate only through one-step reaction, and has simple process route and great industrial value.
Owner:ZHEJIANG MEDICINE CO LTD +1
Positive electrode active material for non-aqueous electrolyte secondary battery and non-aqueous electrolyte secondary battery
InactiveUS20080233481A1Large capacityIncrease productionPositive electrodesLi-accumulatorsSite occupancyMetal
To provide a positive electrode active material for a non-aqueous electrolyte secondary battery, which can achieve high capacity and high output simultaneously, and a non-aqueous electrolyte secondary battery using the same. A non-aqueous electrolyte secondary battery is obtained by using as a positive electrode, a positive electrode active material for a non-aqueous electrolyte secondary battery, which is expressed by the general formula: Lix(Ni1-yCoy)1-zMzO2 (0.98≦x≦1.10, 0.05≦y≦0.4, 0.01≦z≦0.2, M=at least one element selected from the group of Al, Zn, Ti and Mg), and which has a Li site occupancy of the Li site in crystal of 98.5% or more, and a metal site occupancy of the metal site of from 95% to 98% inclusive, obtained by Rietveld analysis.
Owner:SUMITOMO METAL MINING CO LTD +1
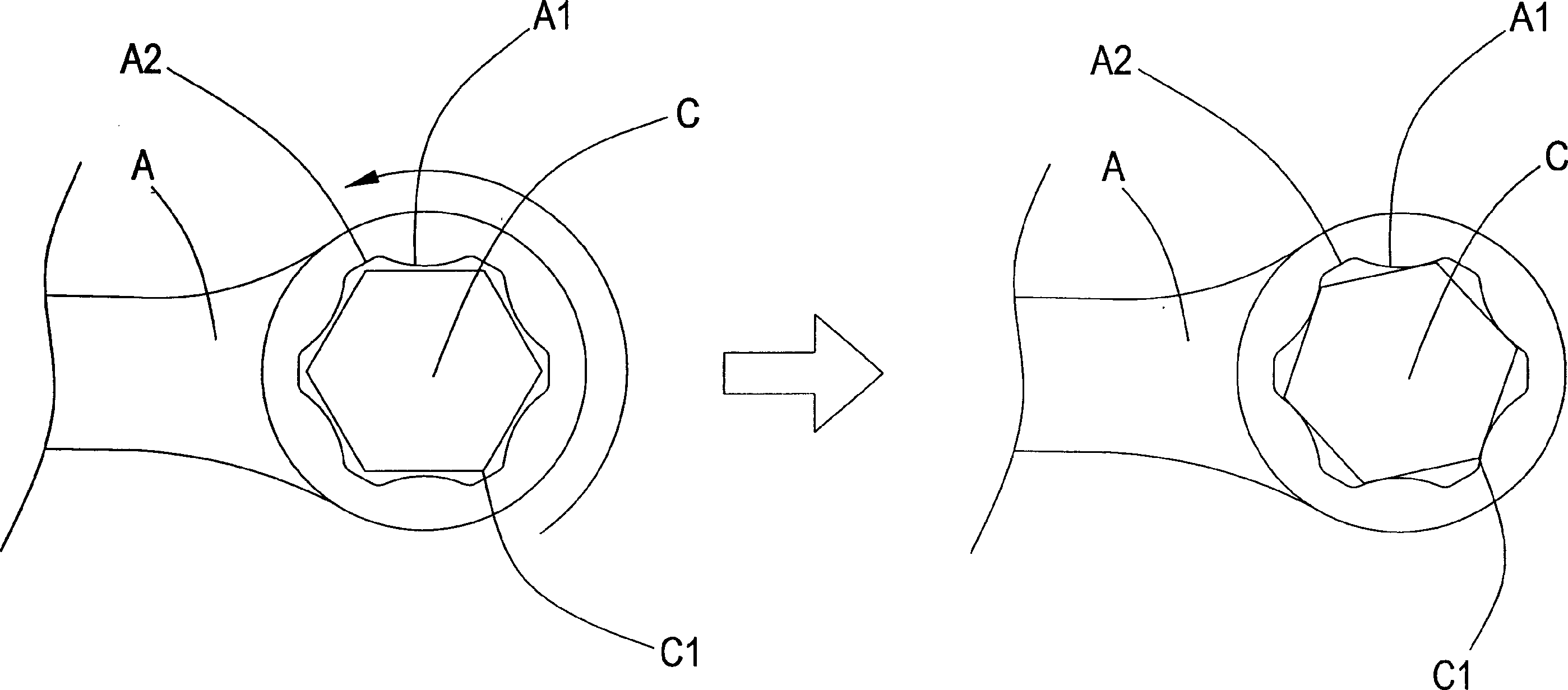
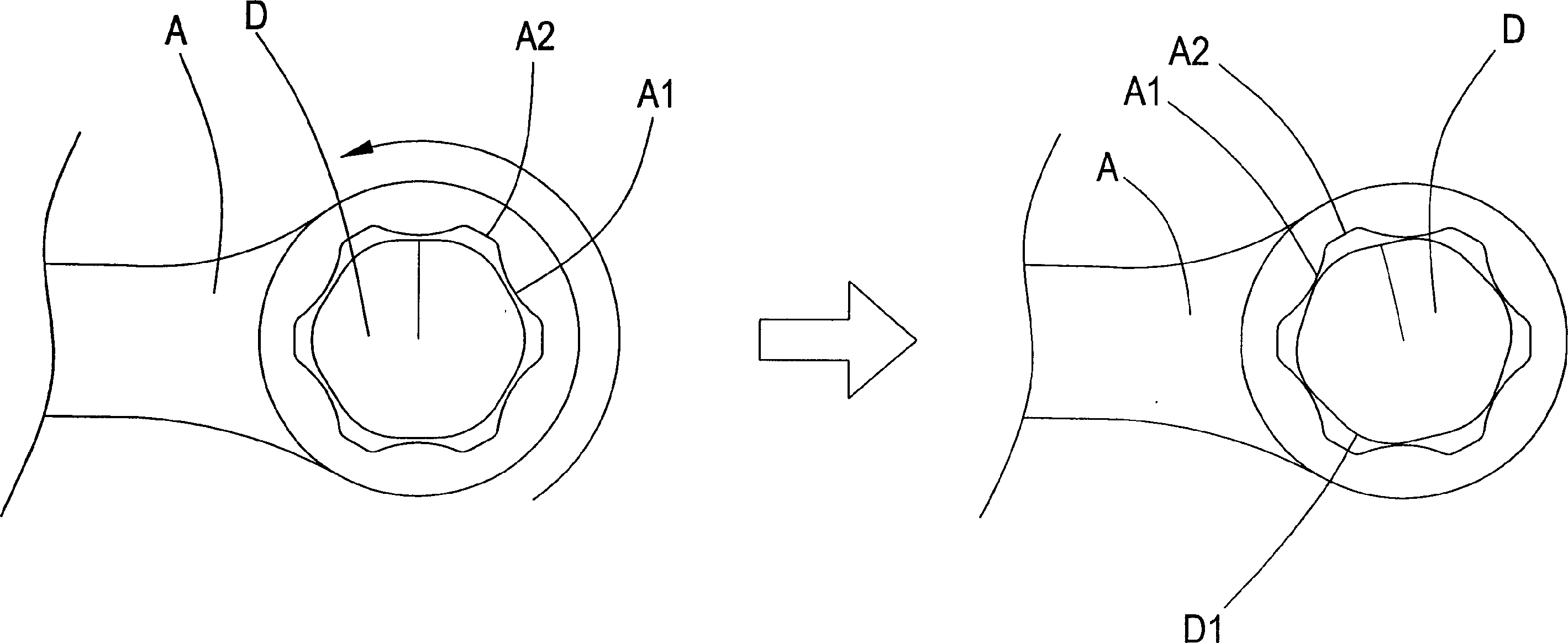
Spanner adapted for highly worn screw nut
InactiveCN1481973AEffective dispersionImprove stabilitySpannersWrenchesScrew capBiomedical engineering
Owner:谢智庆
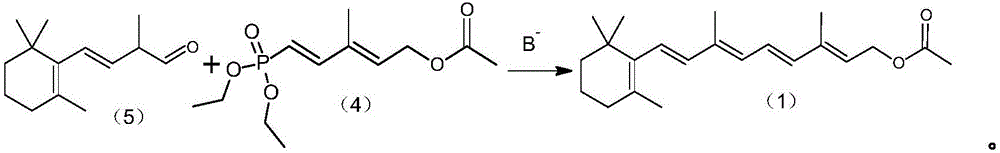
Preparation method of vitamin A acetate
ActiveCN105949101ASimple process routeMild reaction conditionsGroup 5/15 element organic compoundsDiethyl phosphateButene
The invention provides a preparation method of vitamin A acetate. The preparation method comprises the following steps: (1) under an alkaline condition, enabling 3-methyl-4-oxo-2-buten-1-base acetate (short for C5 aldehydo-ester) and tetraethyl methylenediphosphonate to produce a condensation reaction to obtain an intermediate, namely C6 phosphate (be chemically described as 5-(phosphoric acid diethyl ester base)-3-methyl-2,4-hexadiene-1-acetate); (2) carrying out transposition on the C6 phosphate under the alkaline condition, adding C14 aldehyde (be chemically described as 2-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-butene-1-aldehyde) to produce a Wittig-Horner condensation reaction to generate the vitamin A acetate. The preparation method is easy in raw material obtaining, short in the synthetic route, safe in operation and suitable for industrial production.
Owner:肇庆巨元生化有限公司
Fusing mechanism of packer
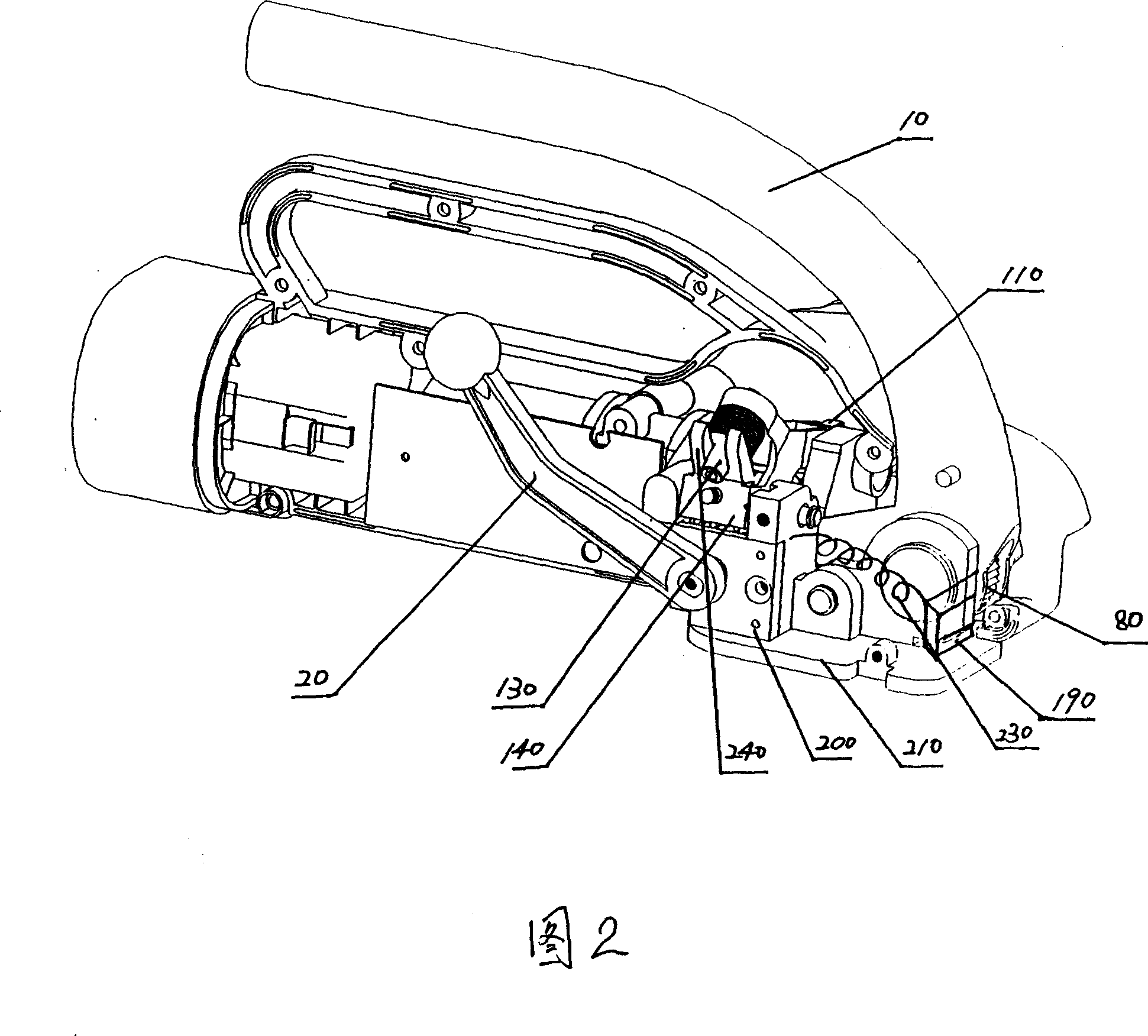
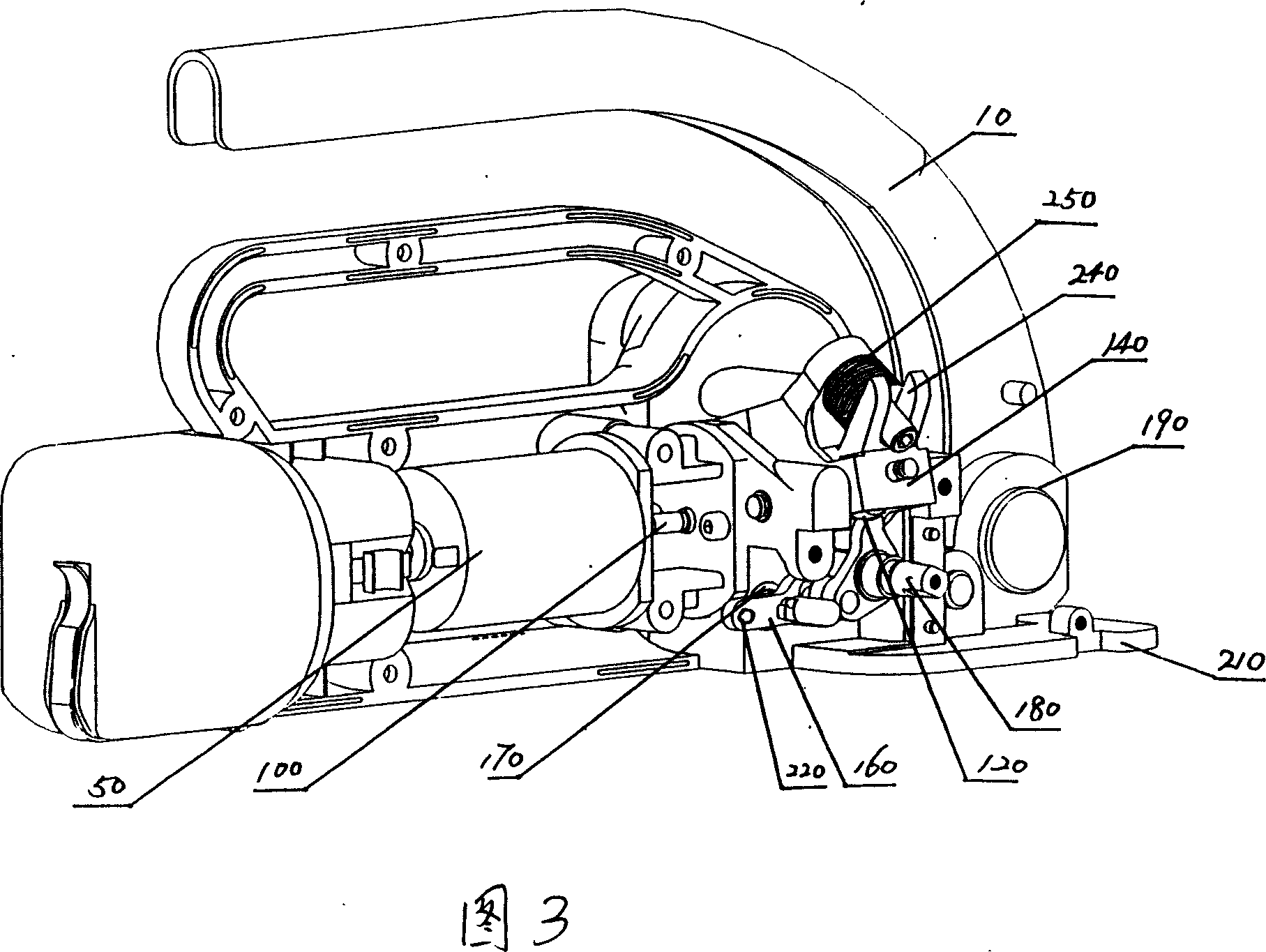
InactiveCN101092187AReasonable workmanshipIngenious ideaBinding material applicationBundling machine detailsEngineering
Owner:SHANGHAI LIYI ELECTRIC
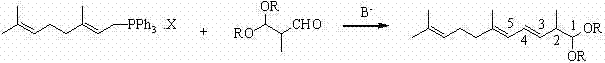
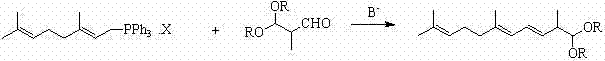
Method for preparing 2,6,10-trimethyl-1,1-dialkoxyl-3,5,9-undecatriene
ActiveCN102311320ASimple process routeRaw materials are easy to getOrganic chemistryOrganic compound preparationOrganic solventLycopene
The invention relates to a method for preparing 2,6,10-trimethyl-1,1-dialkoxyl-3,5,9-undecatriene, belonging to the technical field of lycopene intermediate synthesis methods. The method comprises the following steps: (1) under the protection of inert gas and in the presence of an organic solvent and alkali, carrying out dissociation reaction on C10 triphenyl phosphonium salt at the temperature of minus 40-30 DEG C; and (2) after the dissociation reaction in the step (1) is completed, adding C4 acetal, carrying out Wittig condensation reaction at the temperature of minus 40-30 DEG C in the presence of the organic solvent and alkali so as to obtain 2,6,10-trimethyl-1,1-dialkoxyl-3,5,9-undecatriene. The method provided by the invention has the advantages of concise process route and easy obtainment of raw materials, low cost and high industrial value.
Owner:启东欧常新材料有限公司
Method for purifying lactobacillus rhamnosus extracellular polysaccharides
InactiveCN106011196APromote growthEnhanced inhibitory effectMicroorganism based processesFermentationFreeze-dryingLactobacillus rhamnosus
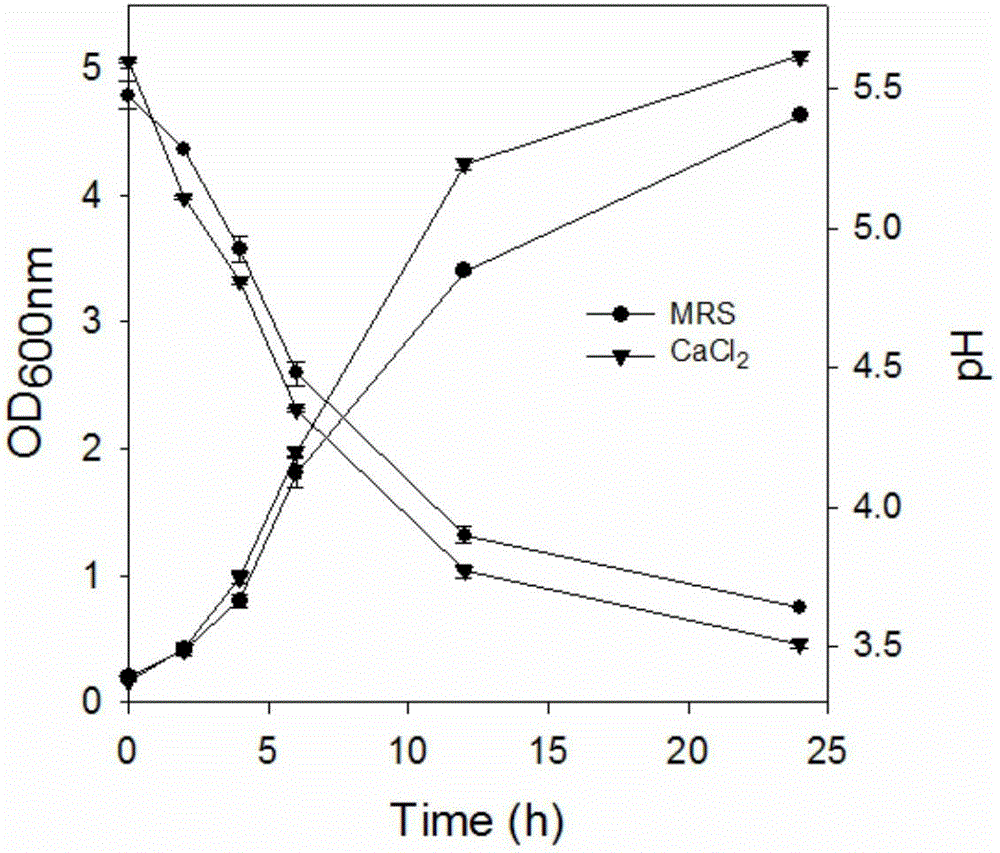
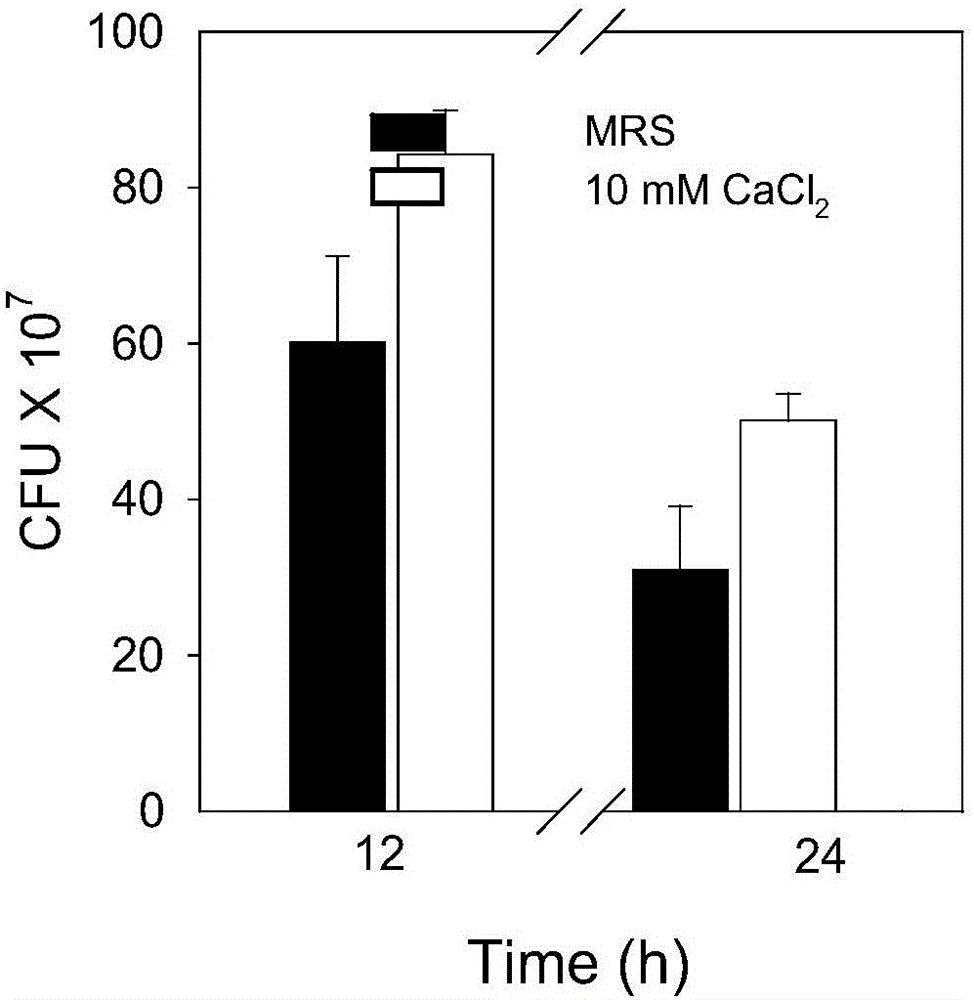
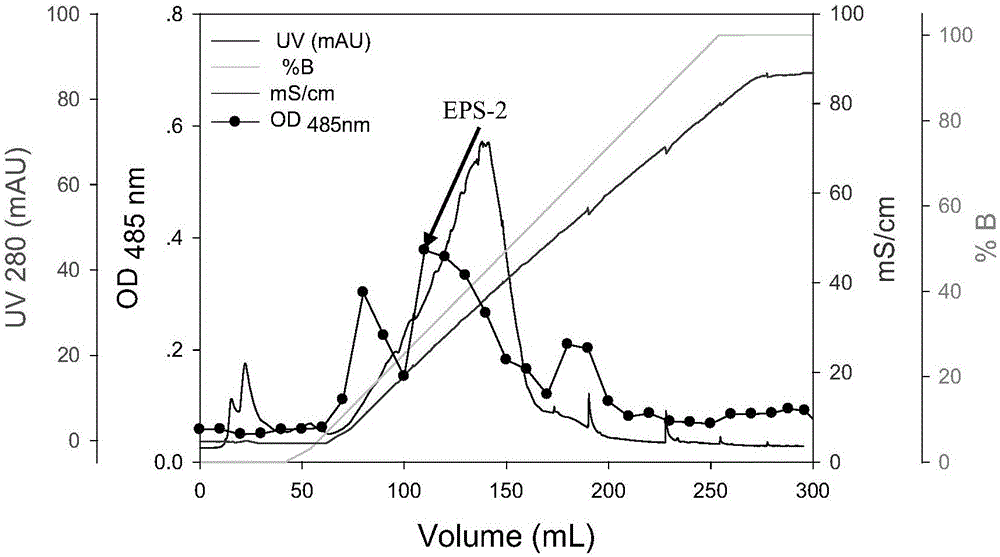
The invention discloses a method for purifying lactobacillus rhamnosus extracellular polysaccharides and relates to the extracellular polysaccharides. The method includes: inoculating a single colony on the MRS solid culture medium of seed conservation to the activated fermentation liquid cultured in the MRS liquid culture medium; adding CaCl2 with the final concentration being 10mM into the MRS liquid culture medium, using the activated fermentation liquid for inoculation to obtain inoculated fermentation liquid, and measuring the OD600 and pH values of 1ml of inoculated liquid which is cultured for 0, 2, 4, 6, 12 and 24 hours; removing the cells and protein of the inoculated fermentation liquid, adding glacial acetic acid, placing into a refrigerator for one night, centrifuging, removing supernatant, performing freeze-drying on sediment, taking out, dialyzing, performing freeze-drying to obtain crude extracellular polysaccharides, dissolving into pure water, dissolving the extracellular polysaccharides sample after dialyzing and freeze-drying into distilled water to prepare a 20mg / mL solution, centrifuging, and performing ion column chromatography on supernatant to obtain the lactobacillus rhamnosus extracellular polysaccharides.
Owner:XIAMEN UNIV
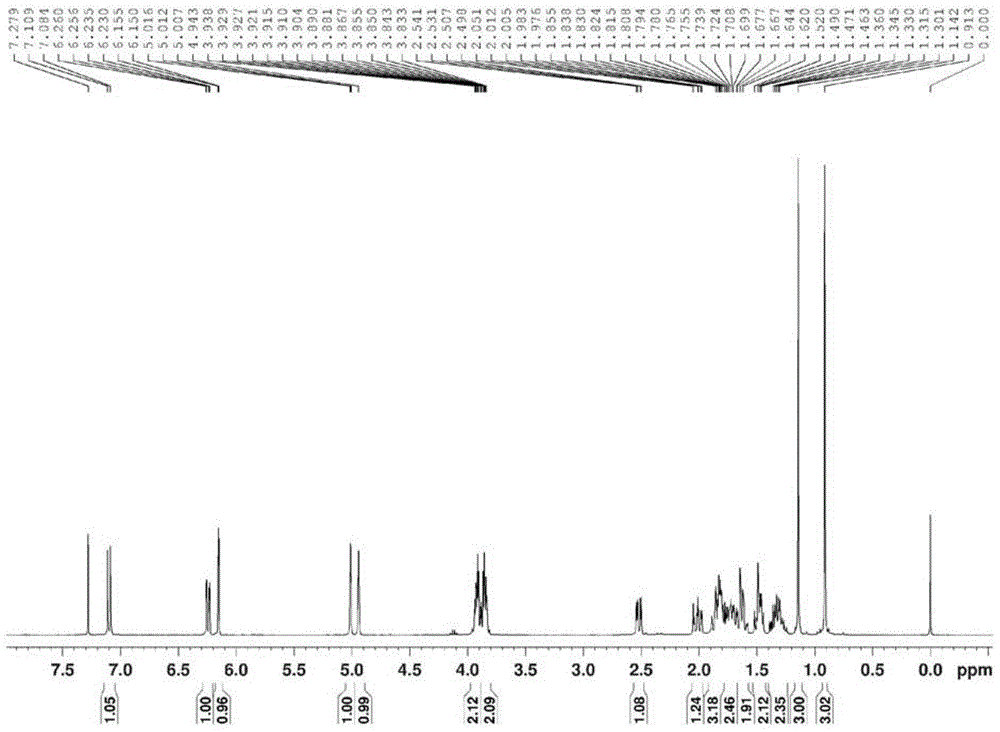
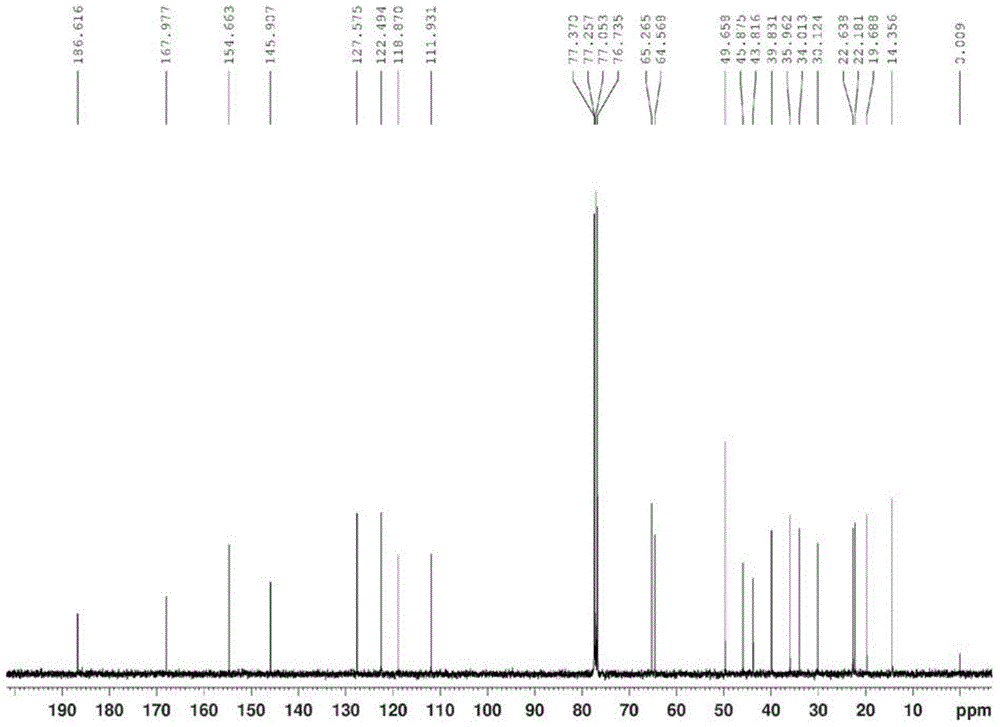
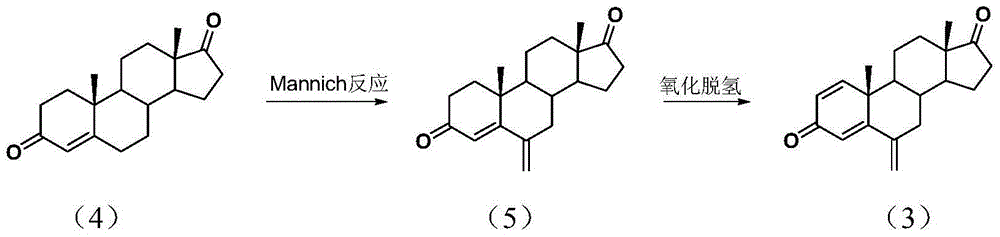
Exemestane intermediate 17,17-ethyldioxy-6-methyleneandrost-1,4-diene-3-ketone and preparation method and application thereof
InactiveCN105085599ASimple process routeRaw materials are easy to getKetal steroidsMannich reactionIsoamyl alcohol
The invention discloses an exemestane intermediate 17,17-ethyldioxy-6-methyleneandrost-1,4-diene-3-ketone and its preparation method and application. Most existing methods have low overall yield and are not suitable for industrial production. The invention provides an exemestane intermediate 17,17-ethyldioxy-6-methyleneandrost-1,4-diene-3-ketone with a new structural formula. The preparation method comprises the following steps: firstly, dimethylamine hydrochloride and paraformaldehyde undergo reflux carrying water in isoamyl alcohol; and then, androstadienone ethylene glycol ketal is added to carry out an Mannich reaction so as to prepare 17,17-ethyldioxy-6-methyleneandrost-1,4-diene-3-ketone, and 17,17-ethyldioxy-6-methyleneandrost-1,4-diene-3-ketone is hydrolyzed to prepare exemestane. The method is simple and has industrial value.
Owner:SHAOXING UNIVERSITY +1
Technique for manufacturing packer with fusing mechanism
InactiveCN101092186AReasonable workmanshipIngenious ideaBinding material applicationBundling machine detailsManufacturing technologyControl theory
The invention discloses packer manufacturing technology. It includes the following steps: setting compacting arm in the wall board; setting compacting adjusting piece on the arm; setting the press roller on the adjusting piece; setting arched spring on the compacting adjusting screw; setting the press belt piece connecting lever on the compacting arm by pin; connecting the oscillating arm with eccentric shaft of the direct current machine. The invention can use the heat quantity caused by packing belt motion with high speed to realize self actuated fusion while packing. Thus it has great industry utility value.
Owner:SHANGHAI LIYI ELECTRIC
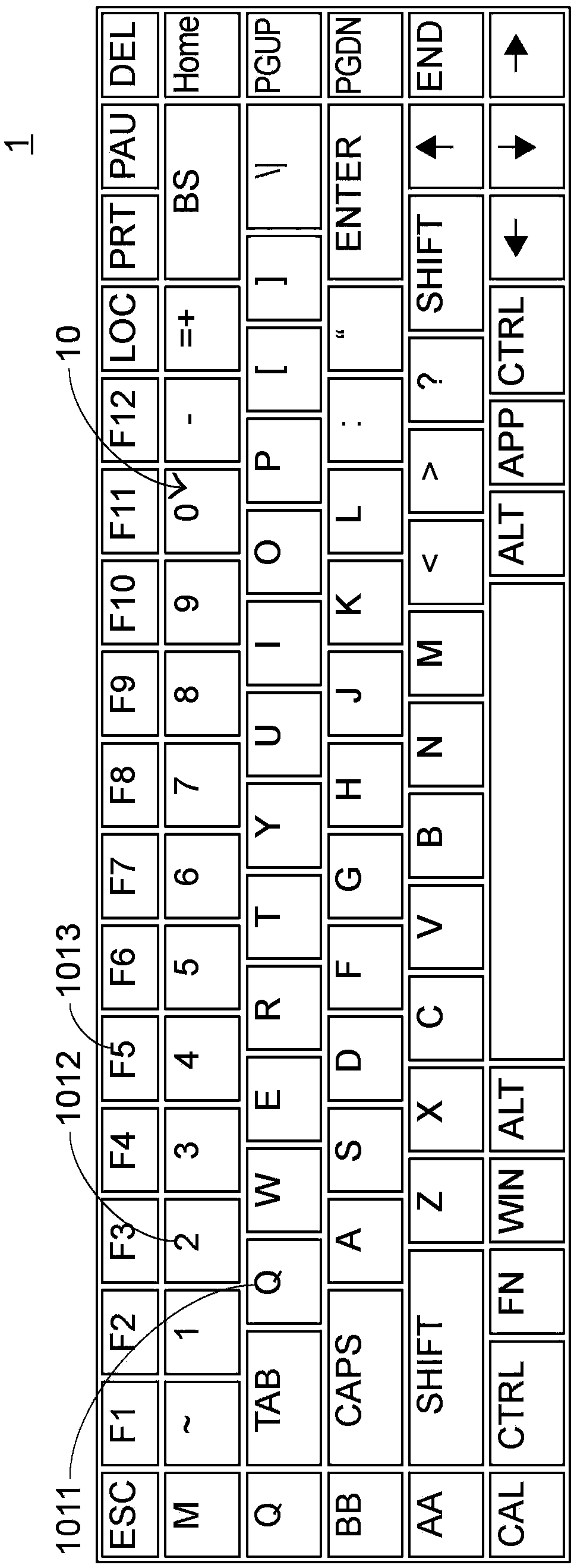
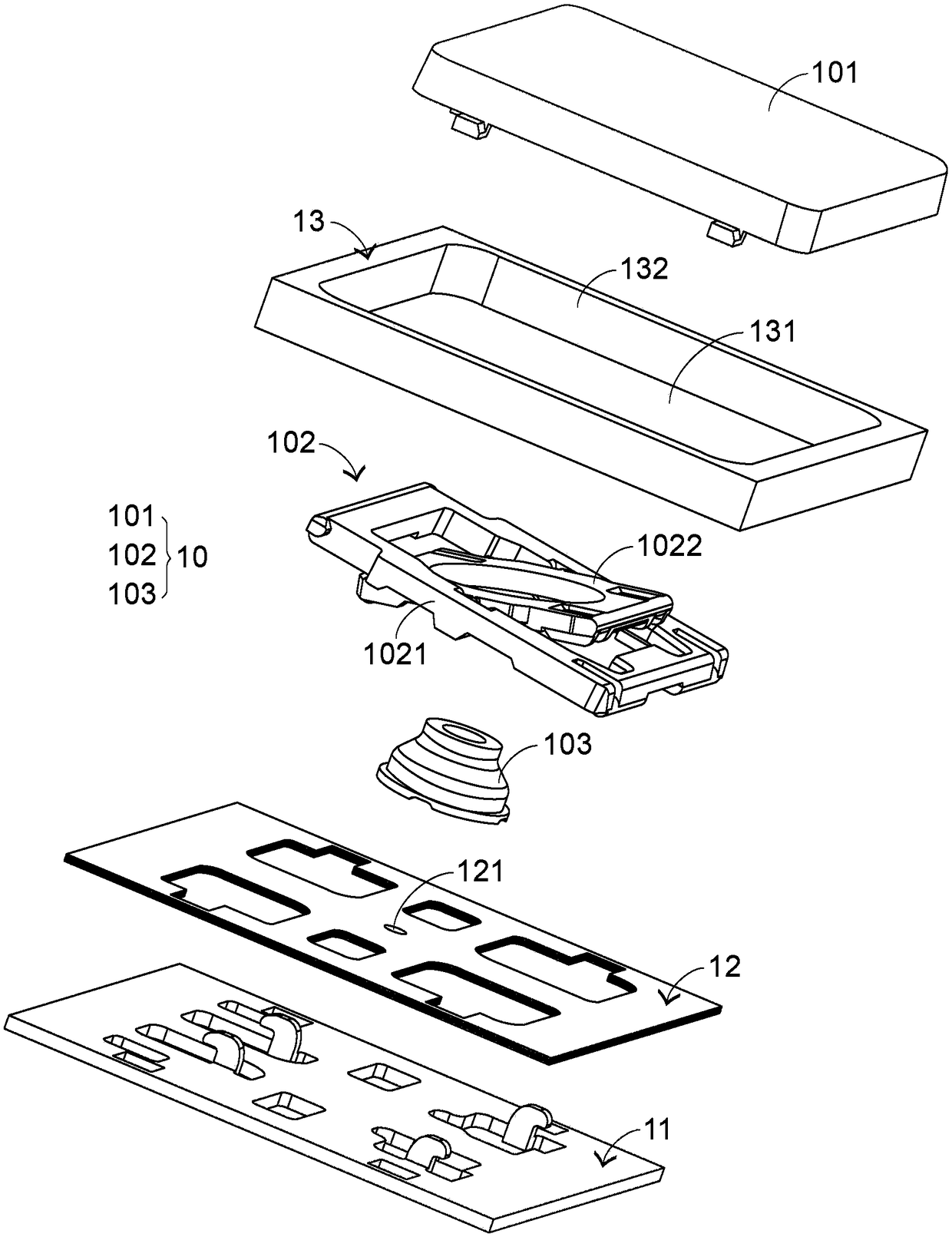
Keyboard device
InactiveCN108958492AIndustrial valueSimplify the tedious processInput/output for user-computer interactionLegendsKey pressingLight beam
The invention provides a keyboard device comprising a plurality of key structures, an electronic paper display element and a light-emitting module. The electronic paper display element is arranged below a plurality of key structures and used for displaying a plurality of key symbols corresponding to the plurality of key structures. The light-emitting module is arranged on the electronic paper display element and provides light beams to the electronic paper display element to illuminate the plurality of key symbols.
Owner:PRIMAX ELECTRONICS LTD
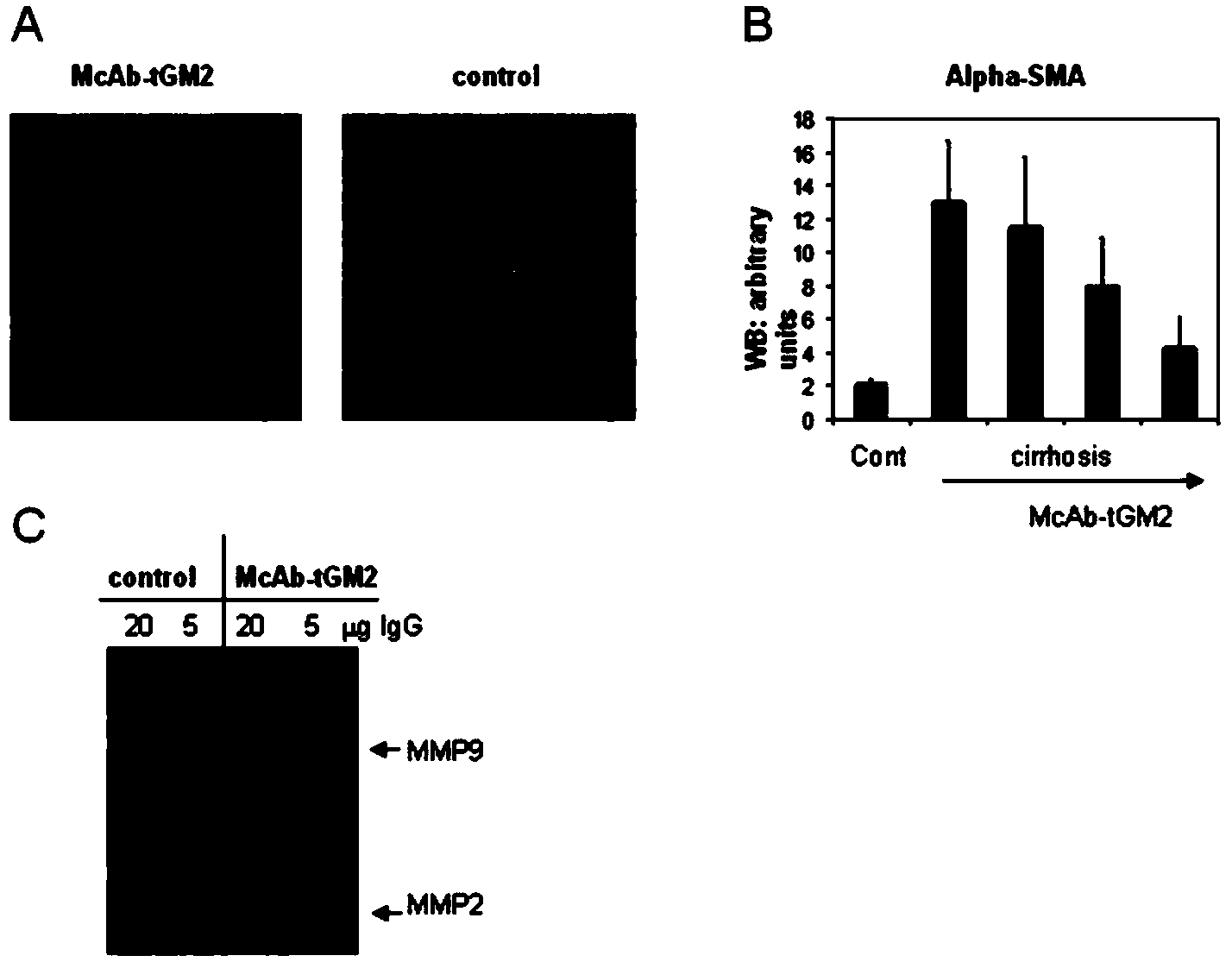
Anti-human tissue transglutaminase 2 monoclonal antibody and application thereof
InactiveCN104278013APrevent proliferationPromote degradationDigestive systemMicroorganism based processesGlutaminase 2Tissue transglutaminase
The invention discloses an anti-human tissue transglutaminase 2 monoclonal antibody and application thereof. A rat hybridoma cell strain, particularly rat anti-human tissue transglutaminase 2 (tGM2) hybridoma cell XK-1 (CCTCC NO:C201312), is established. The hybridoma cell secretes an anti-human tissue transglutaminase 2 (tGM2) monoclonal antibody; and the monoclonal antibody protein has a specific inhibition action on tissue transglutaminase 2 (tGM2), is capable of effectively inhibiting enzyme activity of mouse tGM2 and restoring activity of matrix metal proteinases (MMPs), and has the function of treating liver fibrosis.
Owner:WUHAN XIEKANG BIOTECH
Method for producing porcine alpha-interferon
InactiveCN102559819AHigh purityHigh activityMicroorganism based processesPeptide preparation methodsInterferon alphaHigh activity
The invention relates to a method for producing porcine alpha-interferon, and belongs to the field of the treatment of porcine infection diseases. The invention mainly provides a method for secreting, separating and purifying interferon simply, and aims to simplify a production process, reduce production cost and obtain a recombinant porcine alpha-interferon product with high activity and high stability. The production method comprises the following steps of: producing interferon by using a mixture of methanol; purifying the interferon by multiple-effect ultrafiltration; and making ultrafiltration membranes with different molecular weight cutoff according to the molecular weight of the interferon into a filter, and filtering, so that impure protein and impurities of which the molecular weight is greater than that of the interferon and foreign protein, inorganic salt and water of which the molecular weight is smaller than that of the interferon in a fermentation liquor are removed, and only the interferon and little water are reserved; and freezing and drying to obtain an interferon finished product which is high in purity, activity and stability.
Owner:GUANGDONG ZIJIN ZHENGTIAN PHARMA
Method for preparing alcohol through hydroformylation of olefin which is directly regenerated and reused by cobalt catalyst
InactiveCN109020791ASimplify the production processReduce equipment costsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemistryPre treatment
The invention discloses a method for preparing alcohol through hydroformylation of olefin which is directly regenerated and reused by a cobalt catalyst. According to the method, a deactivated catalyzing system can recover reaction activity through pretreatment, an intermittent type reaction kettle is utilized, reaction pressure is 4 to 18 MPa, a temperature is 170 to 210 DEG C, and CO and H2 mixedgas is utilized to react. A deactivated catalyst pretreatment work procedure is performed in a reaction kettle under the CO and hydrogen mixed condition. The method has a simple technology, can greatly reduce production cost and can improve production efficiency.
Owner:广东仁康达材料科技有限公司
1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-1,3-butadiene and preparation method thereof
InactiveCN104418713ASimple process routeRaw materials are easy to getOrganic compound preparationCarbonyl compound preparation by hydrolysisCyclohexeneEnol ether
The invention provides an enol ether 1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-1,3-butadiene shown in a formula I and a preparation method thereof. In the presence of an acid catalyst, the 1-methoxy-2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-1,3-butadiene is subjected to hydrolysis reaction to prepare 2-methyl-4-(2,6,6-trimethyl-2-cyclohexene-1-yl)-2-butene-1-aldehyde. The process line is simple, easily available in raw materials and low in cost, and has industrial value.
Owner:SHAOXING UNIVERSITY +1
Lever manipulator
InactiveCN104287911ASimple structureIncrease production capacityWheelchairs/patient conveyanceEngineeringManipulator
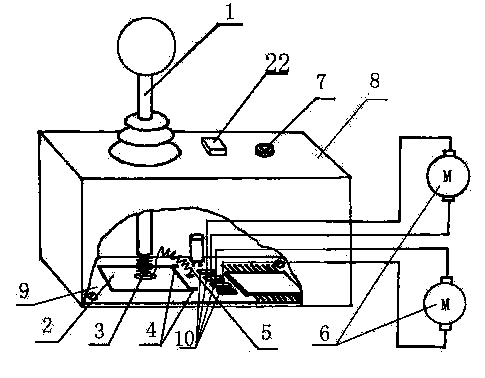
The invention discloses a lever manipulator which comprises a control handle, a sensing element and a sensing source. The lever manipulator is characterized in that at least one sensing element and at least one sensing source are included, the sensing element, the sensing source and the control handle together form a device which outputs two change signals by adjusting the positions of the sensing source and the sensing element. According to the lever manipulator, fewer elements are used, and the defect that reliability is reduced is overcome.
Owner:过志浩
Ceramics with excellent electrostrictive property
InactiveUS6093667AExcellent electrostrictive propertySmall hysteresisPiezoelectric/electrostrictive device material selectionCeramic shaping apparatusSolid solutionCeramic
The present invention provides novel ceramic materials with excellent electrostrictive property, and the present invention relates to electrostrictive ceramics consisting of solid solution ceramics which can be obtained by combining about 30 molar % of primitive perovskite-type compound PbTiO3 with a composite perovskite compound Pb(Ni1 / 3Nb2 / 3)O3.
Owner:NAT INST OF ADVANCED IND SCI & TECH
LCD disply device and manufacture thereof
ActiveCN1854871AEnsure electrical connectionLow costTransistorStatic indicating devicesLiquid-crystal displayInsulation layer
A method for preparing liquid crystal display device includes utilizing semitone image exposure technique to semiconductor layer of insulation grid film transistor and source / drain electrode wiring on primary photo-etching process, setting signal wire and analog pixel electrode in stacked mode between transparent conduction layer and low resistance metal, removing off low resistance metal layer on said pixel electrode to obtain pixel electrode formed by transparent conduction layer when opening is formed on passivated insulation layer, removing off grid insulation layer to obtain contact point properly when semiconductor layer is formed.
Owner:QUANTA DISPLAY JAPAN +1
Preparation method of 4-amino-benzoyl formic-N-(4-amino-benzoyl formic) amine
ActiveCN101870664BHigh purityIndustrial valueOrganic compound preparationCarboxylic acid amides preparationHydrazine compoundSolvent
The invention relates to a preparation method of 4-amino-benzoyl formic-N-(4-amino-benzoyl formic) amine, which belongs to the technical field of the compound preparation method. The invention particularly relates to a preparation method of an organic pigment intermediate, which is characterized by comprising the following steps that: Para Amino Benzamide and paranitrobenzoyl chloride have condensation reaction in solvent under the condition that alkali carbonate exists to obtain 4-nitrobenzoyl-N-(4-nitrobenzoyl)amine; the 4-nitrobenzoyl-N-(4-nitrobenzoyl)amine is catalyzed and reduced by hydrazine hydrate in the solvent to obtain 4-amino-benzoyl formic-N-(4-amino-benzoyl formic) amine. So far, the preparation method not only improves the purity of the product to more than 99.7 percent, but also improves the total yield to more than 93.2 percent, and improves the quality and the yield of the product. During the synthesis process, the productivity of three wastes is reduced to less than 2 tons of waste water every one ton of product, so the process has industrialized value.
Owner:淄博圣马化工有限公司
Preparation method of C5 Grignard reagent
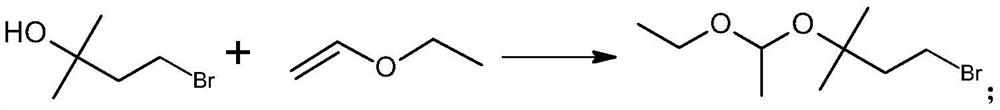
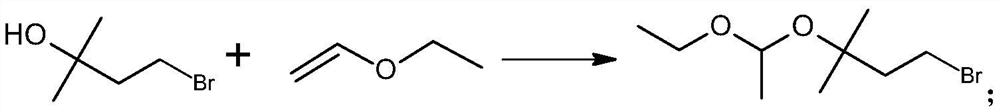
PendingCN113717203ASimple process routeMild reaction conditionsMagnesium organic compoundsPtru catalystGrignard reagent
The invention discloses a preparation method of a C5 Grignard reagent. The preparation method comprises the following steps of: (1) carrying out a hydroxyl acetalization reaction on 2-methyl-2-butanol-4-bromine and vinyl ethyl ether in an organic solvent under the action of a catalyst to obtain 1-bromo-3-(1-ethoxyethoxy)-3-methylbutane; and (2) reacting the 1-bromo-3-(1-ethoxyethoxy)-3-methylbutane with metal magnesium in an organic solvent to generate 3-(1-ethoxyethoxy)-3-methylbutane magnesium bromide. The process route is simple, the reaction conditions are mild, and the process operation is easy to implement.
Owner:肇庆巨元生化有限公司
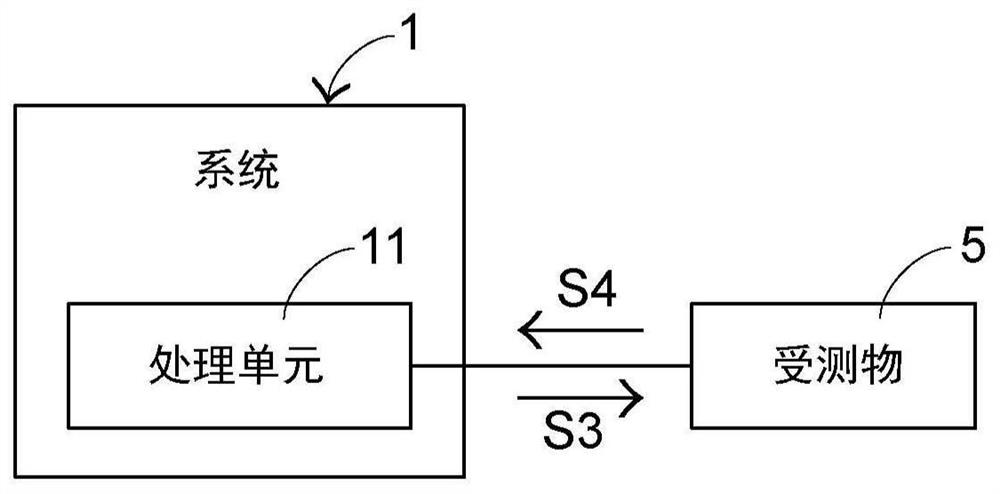
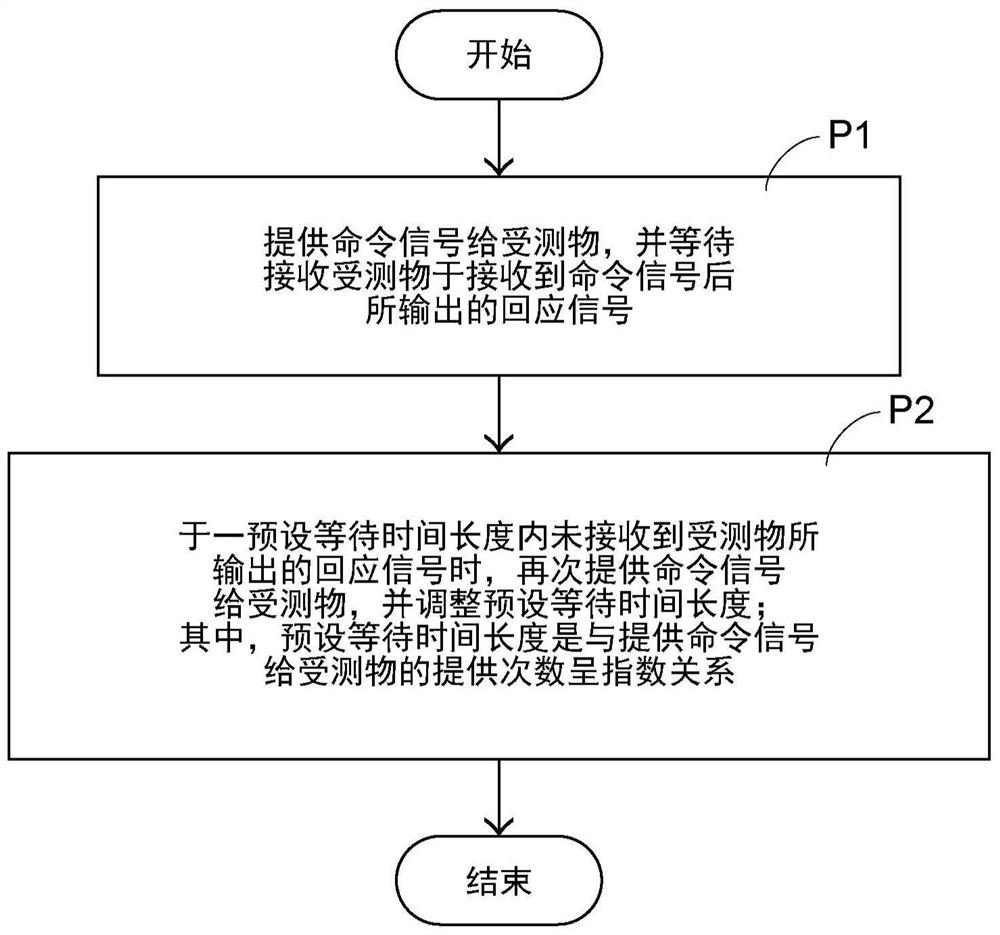
Method for communicating with object under test and system using the method
InactiveCN107544877BReduce chanceReduce the probability of communication failureDetecting faulty computer hardwareFailure rateProcessing element
The present invention provides a method for communicating with an object under test and a system for applying the method. The method of communicating with the object under test includes: providing a command signal to the object under test through the processing unit of the system, and waiting to receive a response signal output by the object under test after receiving the command signal, if the processing unit waits for a preset waiting time length When the response signal output by the object under test is not received, the command signal is provided to the object under test again, and the preset waiting time length is adjusted; wherein, the adjusted preset waiting time length is the same as that of providing the command signal to the object under test The number of times provided has an exponential relationship. The invention can reduce the probability of re-communication and communication failure rate, and shorten the communication time.
Owner:PRIMAX ELECTRONICS LTD
Process of making cutting mechanism of baler
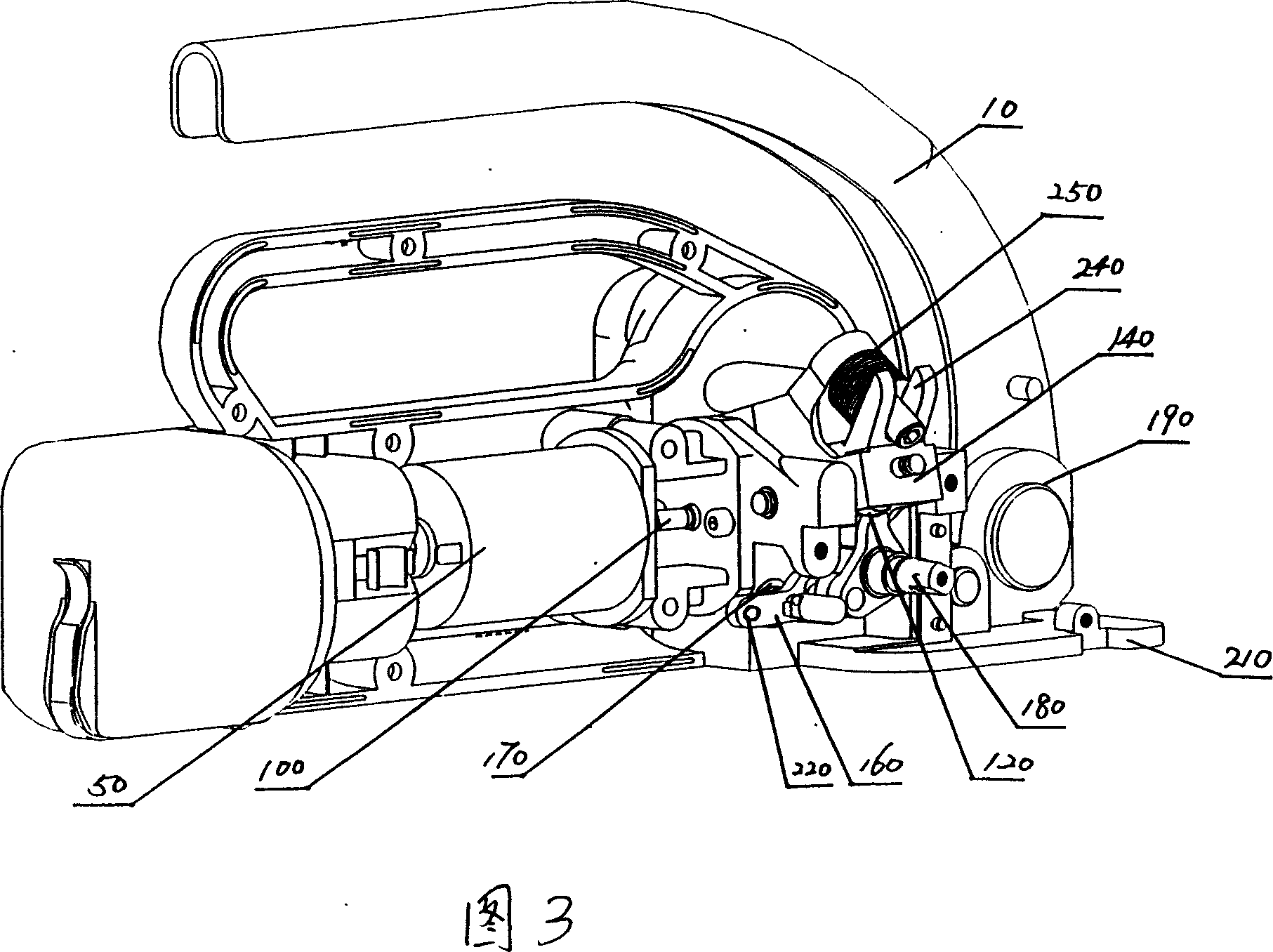
InactiveCN101088869AReasonable workmanshipIngenious ideaBinding material applicationPaper/cardboard containersEngineeringCam
The process of making cutting mechanism of baler includes the following steps: 1. assembling one cutter on the cutter holder and connecting the cutting with the cam shaft through one shift fork; and 2. assembling one cam shaft and one belt cutting handle in its one end. The present invention has reasonable technological process, and the cutting mechanism of baler has fluent cutting and easy maintenance and replacement.
Owner:SHANGHAI LIYI ELECTRIC
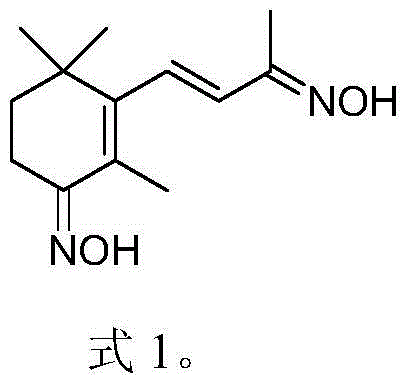
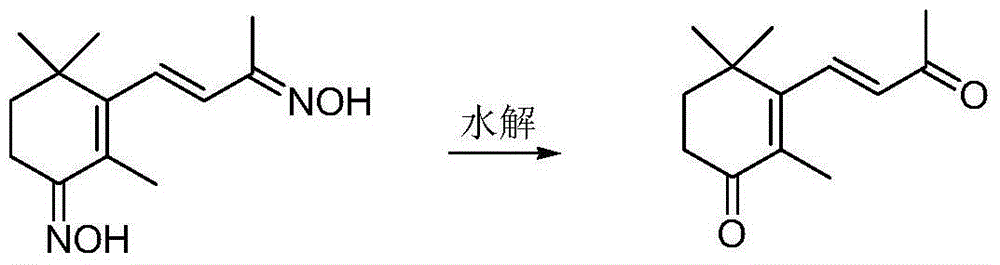
Dioxime ionone as well as preparation method and application thereof
InactiveCN103880705BSimple process routeEasy to operateOximes preparationCarbonyl compound preparation by hydrolysisDouble bondHydroxylamine Hydrochloride
The invention discloses dioxime ionone as well as a preparation method and application thereof, and belongs to the technical field of preparation of pharmaceutical and chemical intermediate products. Dioxime ionone is characterized by being prepared by the steps of oxidizing beta-ionone through oxygen with presence of a catalyst, and implementing an oximation reaction with hydroxylamine hydrochloride to obtain dioxime ionone. The prepared dioxime ionone disclosed by the invention is shown in formula I in the specification, and contains oximido and multi-conjugated double bonds as well as an unique Zhi-ring structure; the dioxime ionone can be further hydrolyzed with presence of an acid to prepare 4-oxo-beta-ionone which is relatively high in purity; therefore, the dioxime ionone has a quite high industrial value.
Owner:SHAOXING UNIVERSITY +1
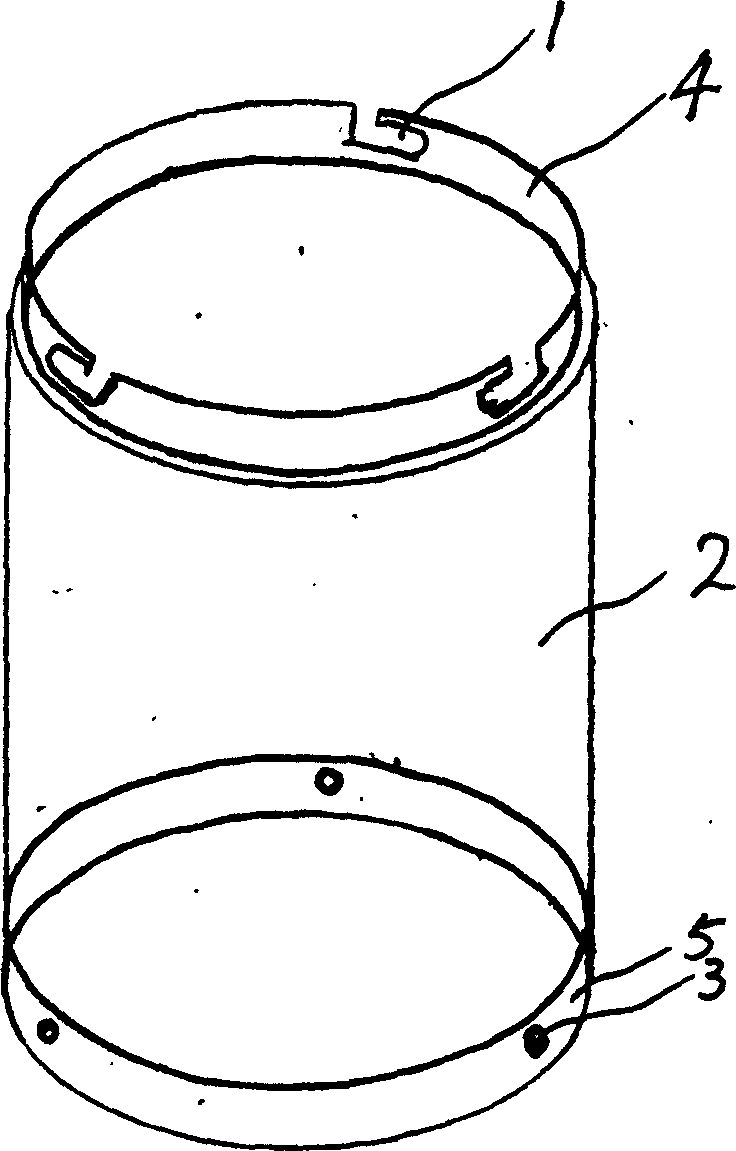
Grain distributor barrel subassembly production process
The invention relates to a method for producing the rice distributing cylinder, wherein it comprises that: a, the upper end of cylinder has one connecting upper edge, with fixing cut; b, the lower end of cylinder has connecting low edge matched with the upper edge, with fixing button matched with fixing cut. The invention has simple structure while the elements can be sheathed to form different capacity.
Owner:上海锦宏自助餐用品制造有限公司
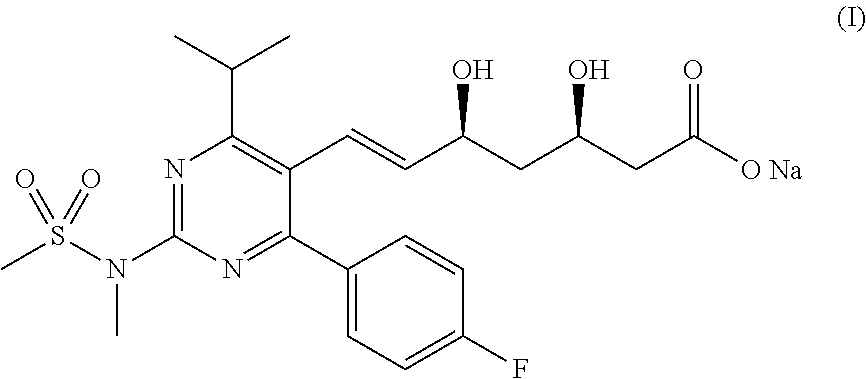
Method for preparing rosuvastatin sodium
ActiveUS9850213B2Efficient methodEasy to getOrganic active ingredientsOrganic chemistryFuranPhosphonium salt
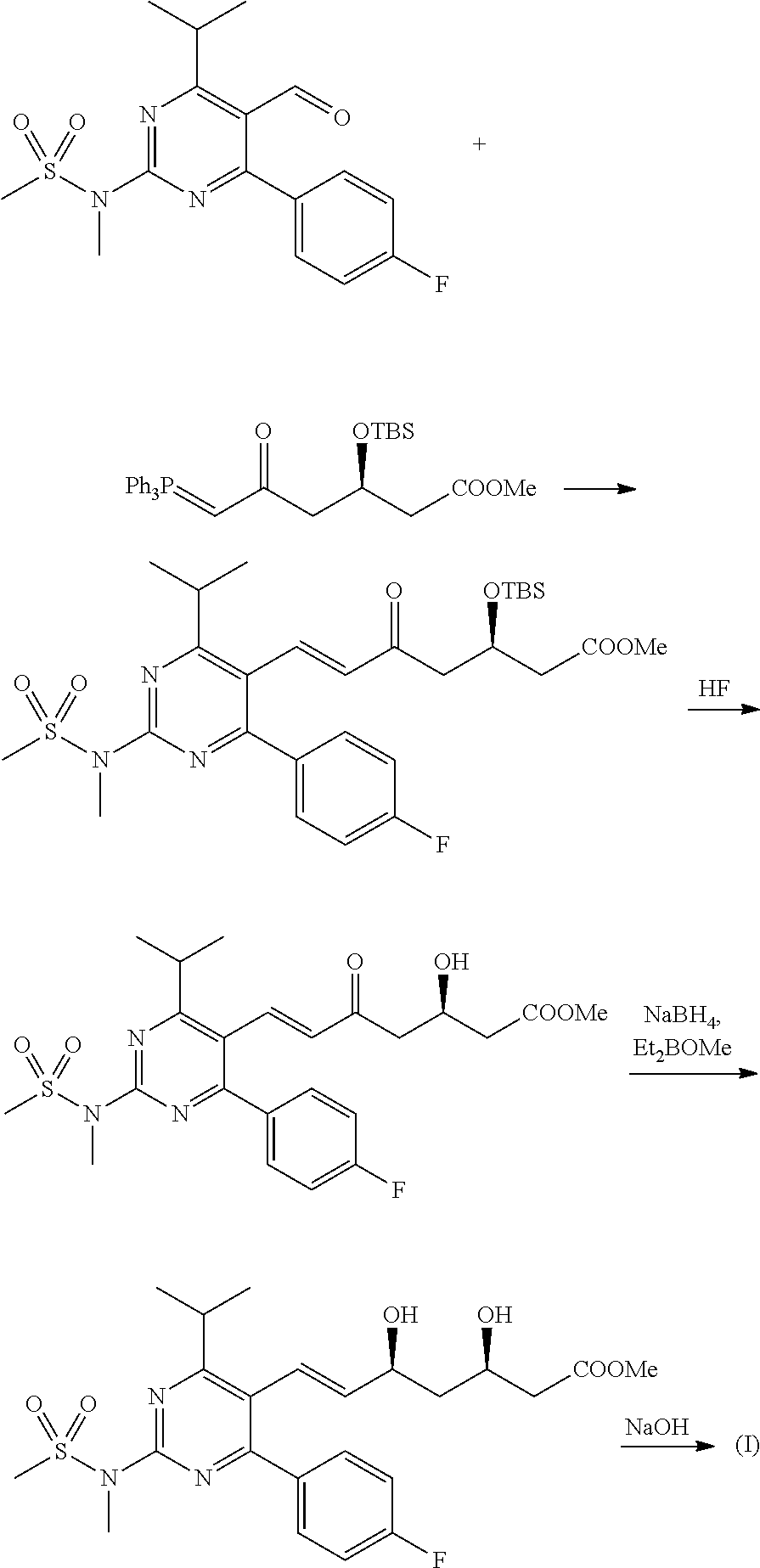
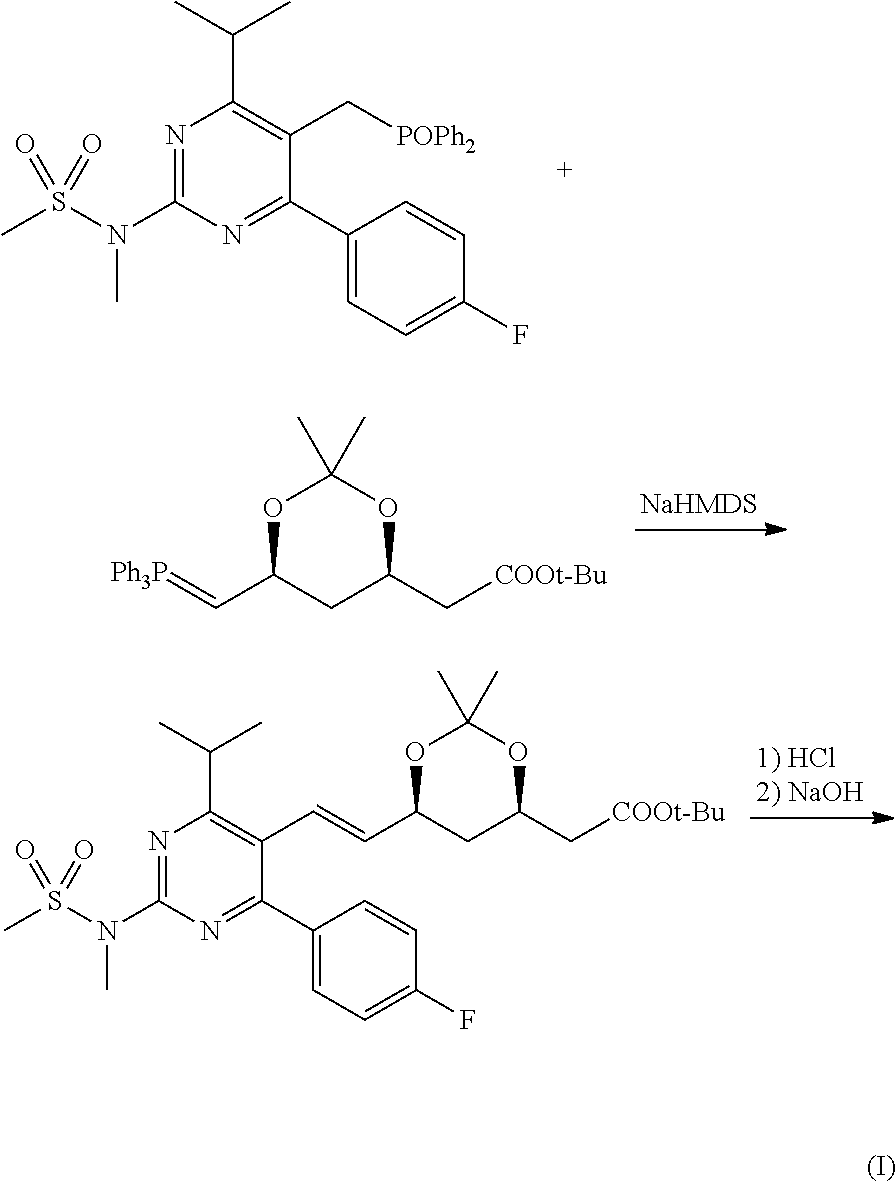
The present invention belongs to the technical field of organic chemistry, and specifically relates to a method for preparing rosuvastatin sodium. The method of the invention comprises: reducing 4-p-fluorophenyl-6-isopropyl-2-(N-methyl-methylsulfonylamino)pyrimidine-5-carboxylic acid (VII) in the presence of a borohydride, an alkyl-substituted chlorosilane and an assistance in an organic solvent to prepare 4-p-fluorophenyl-5-hydroxymethyl-6-isopropyl-2-(N-methyl-methylsulfonylamino) pyrimidine (VIII); then performing a reaction of the compound VIII with a triphenyl phosphonium salt in an organic solvent to prepare a ((4-p-fluorophenyl-6-isopropyl-2-(N-methyl-methylsulfonylamino)-5-pyridyl)-methyl)triphenyl phosphonium salt (IX); performing a stereoselective Michael addition reaction of (S)-trans-4,5-dihydroxy-pent-2-olefine acid ester (II) with furfural (III) to prepare a 2-((4R,6S)-2-(furan-2-yl)-6-hydroxymethyl-1,3-dioxane-4-yl)acetate (IV); oxidizing the compound IV to prepare a 2-((4R,6S)-2-(furan-2-yl)-6-formacyl-1,3-dioxane-4-yl)acetate (V); performing an olefination reaction of the compound V with the (4-p-fluorophenyl-6-isopropyl-2-(N-methyl-methylsulfonylamino)pyrimid-5-yl)-methyl triphenyl substituted phosphonium salt (IX) or phosphate to prepare 2-((4R,6S)-6-(trans-2-(4-p-fluorophenyl-6-isopropyl-2-(N-methyl-methylsulfonylamino)pyrimid-5-yl)vinyl)-2-(furan-2-yl)-1,3-dioxane-4-yl)acetate (VI); and performing deprotection and sodium salt formation of compound VI to prepare rosuvastatin sodium (I). The method has easily obtainable raw materials, and is simple to operate and suitable for industrial productions.
Owner:JIANGXI BOYA SEEHOT PHARMA CO LTD
Technique for manufacturing packer with the tape-cutting handle setup independently
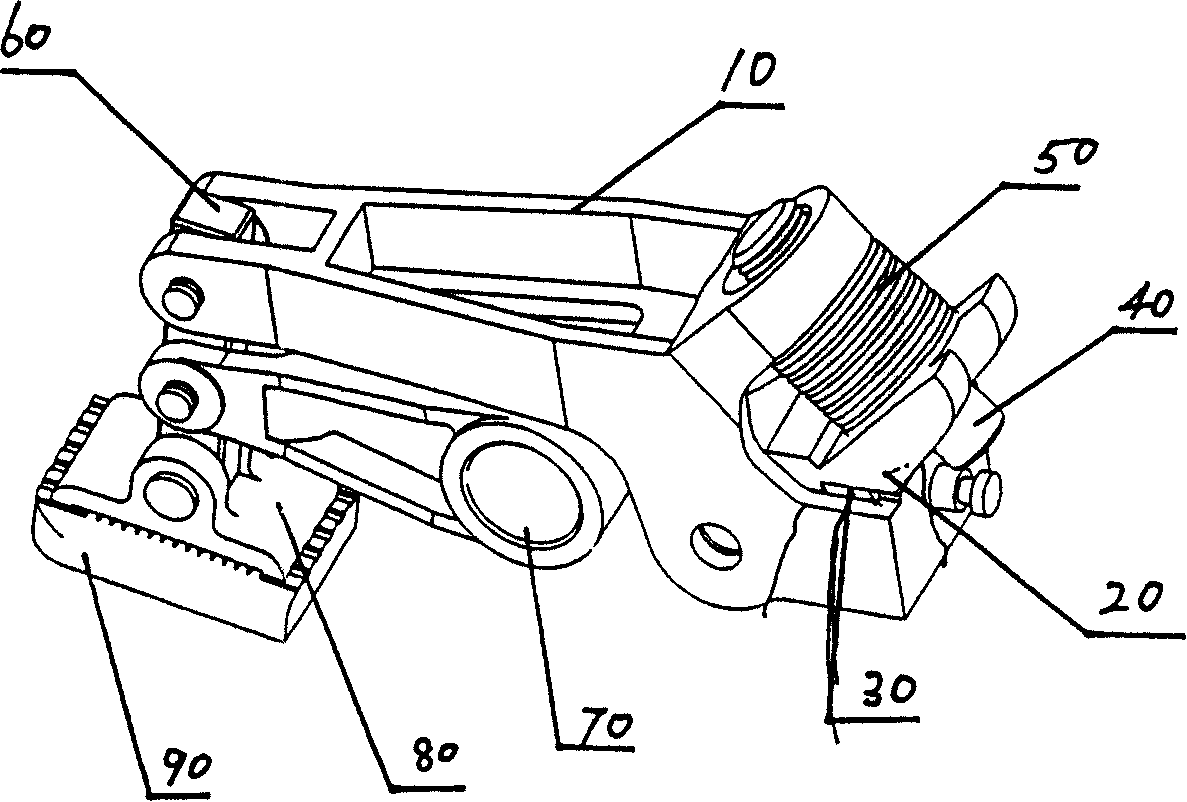
InactiveCN101092172AReasonable workmanshipIngenious ideaBinding material applicationMetal working apparatusIt designCam
The invention discloses a process for making a packer with separately arranged tape cutting handgrip, mainly comprising the step of interlinking the provided tape cutting handgrip with a cam shaft. Its process is reasonable and its design is skillful, and the made packer with separately arranged tape cutting handgrip is convenient to control and has great industrial utilization value because the tape cutting handgrip is separated from tape press and release handgrips.
Owner:SHANGHAI LIYI ELECTRIC
Process of making fusion mechanism of baler
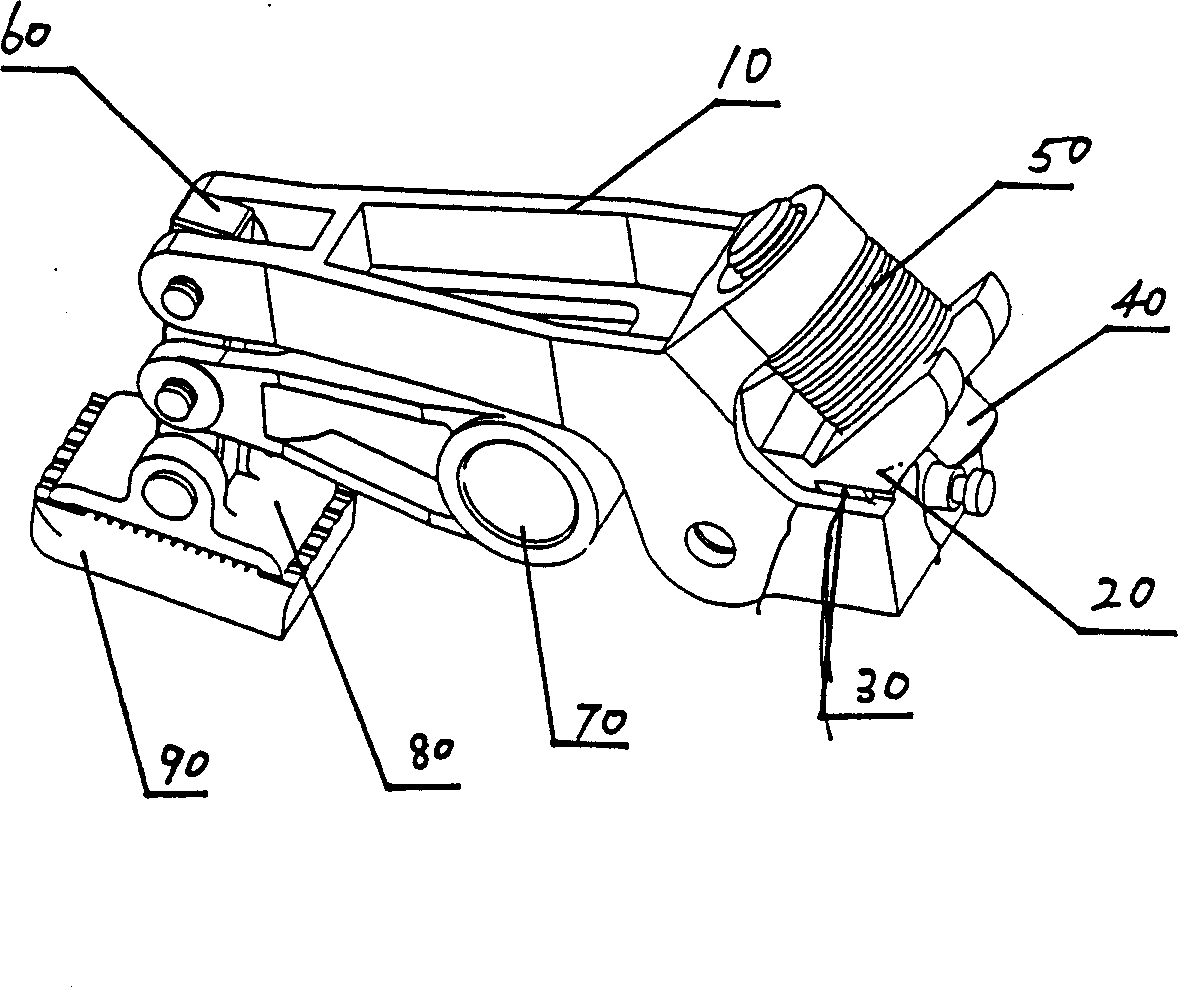
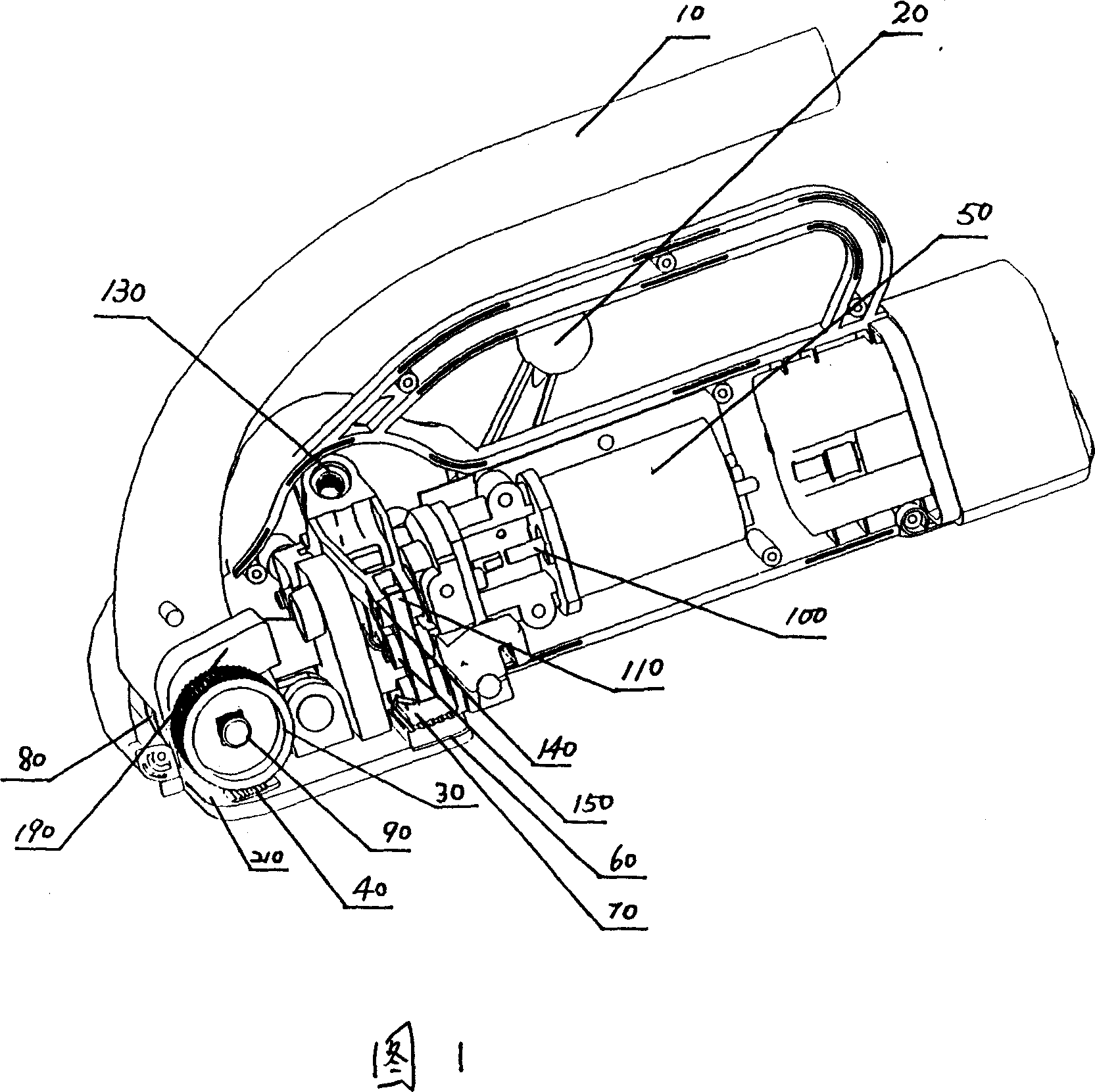
InactiveCN101088871AReasonable workmanshipIngenious ideaBinding material applicationBundling machine detailsFusion mechanismEngineering
The process of making fusion mechanism of baler includes the following steps: 1. assembling one pressing regulator to the pressing arm and one pressing roller with pin, and screwing the pressing regulator screw through the opening in the pressing regulator to the screw hole in the pressing arm; 2. assembling one arced spring to the pressing regulator screw; and 3. assembling one belt pressing unit connecting rod with pin to the pressing arm, assembling one oscillation arm and one swing toothed plate with pin to the belt pressing unit connecting rod, and assembling one fixed toothed plate below the swing toothed plate. The present invention has reasonable technological process, and the made fusion mechanism of baler has self fusion of the baling belt with the friction heat and high marketability.
Owner:SHANGHAI LIYI ELECTRIC
Technique for manufacturing packer with independent setup cutting device
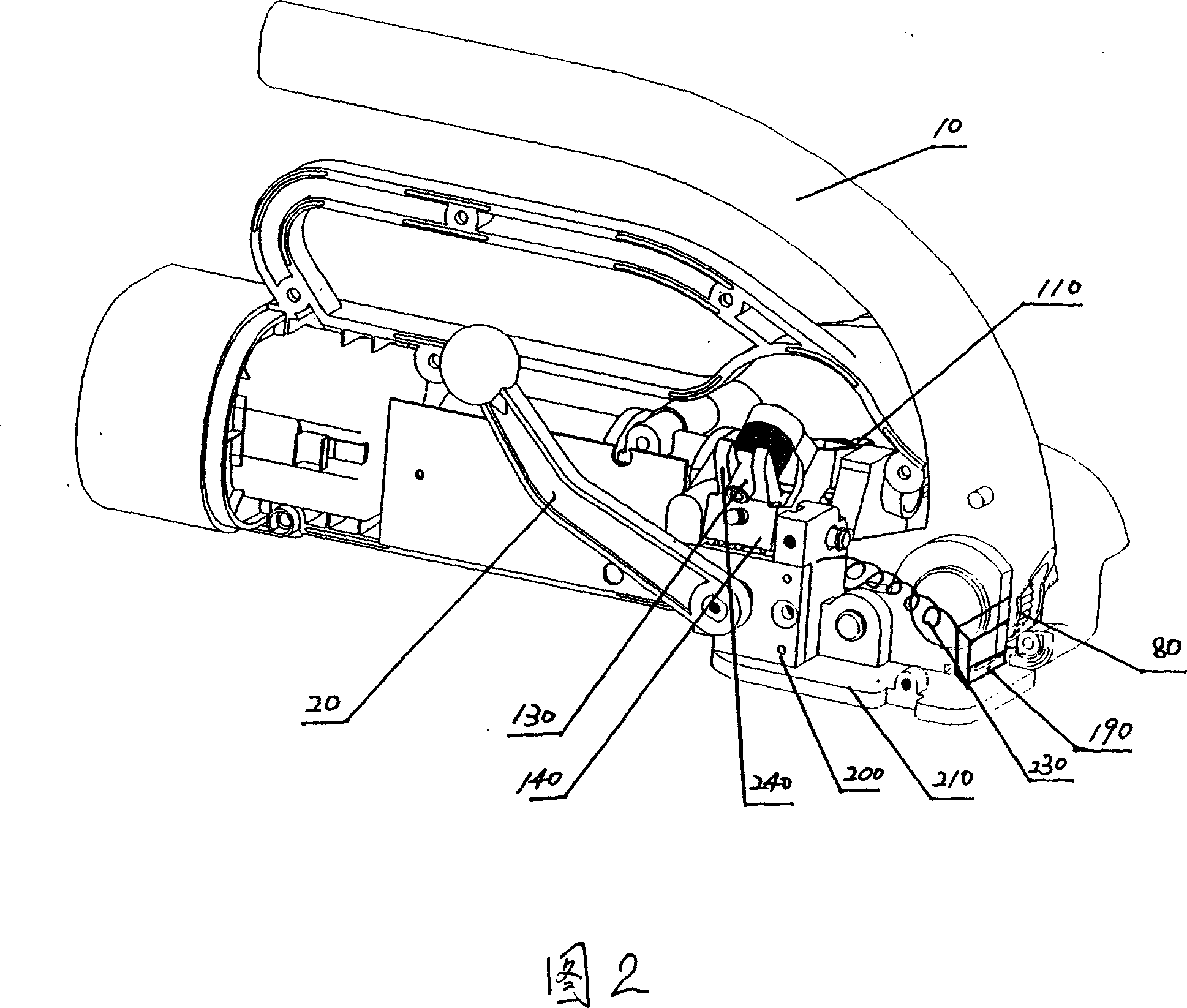
InactiveCN101092173AConducive to matching designReasonable workmanshipBinding material applicationMetal working apparatusEngineeringCamshaft
The invention discloses a packer with separately arranged cutting mechanism, where a cam shaft is provided with tape cutting handgrip, the cam shaft is connected with a cutter on a cutter frame via shift fork, and the cutter is provided with permanent magnet. Its process is reasonable and its design is skillful, and it is beneficial to smoothly completing the cutting procedure, and once the cutting workpiece is damaged, it is convenient to change and maintain, and in addition, it is beneficial to the supporting design of functional diversification of the cutting mechanism and has great industrial utilization value.
Owner:SHANGHAI LIYI ELECTRIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com