Exemestane intermediate 17,17-ethyldioxy-6-methyleneandrost-1,4-diene-3-ketone and preparation method and application thereof
A technology of methylene androster and ethylenedioxy, which is applied in the direction of chemical instruments and methods, steroids, ketal steroids, etc., can solve the problems of low total yield, large pollution, and expensive Jones reagent, and achieve Raw materials are easily available, the process route is simple and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of androstienone ethylene glycol ketal (2)
[0026] Under nitrogen protection, 28.4 grams of 1,4-androstenedione (0.10mol), 14.4 grams of ethylene glycol (0.24mol), 0.4 grams of p-toluenesulfonic acid (0.002mol) and 150 milliliters of toluene were added to 250 milliliters of In a four-necked reaction flask, heated to reflux with water, followed by high-performance liquid chromatography to detect the reaction, and the reaction was completed in about 12 hours. Add 50 ml of aqueous solution containing 1 gram of sodium bicarbonate for neutralization, separate layers, dry over anhydrous magnesium sulfate, recover the solvent under reduced pressure, recrystallize from ethanol to obtain 30.2 grams of light yellow crystals, liquid phase content 99.1%, yield 91.2% .
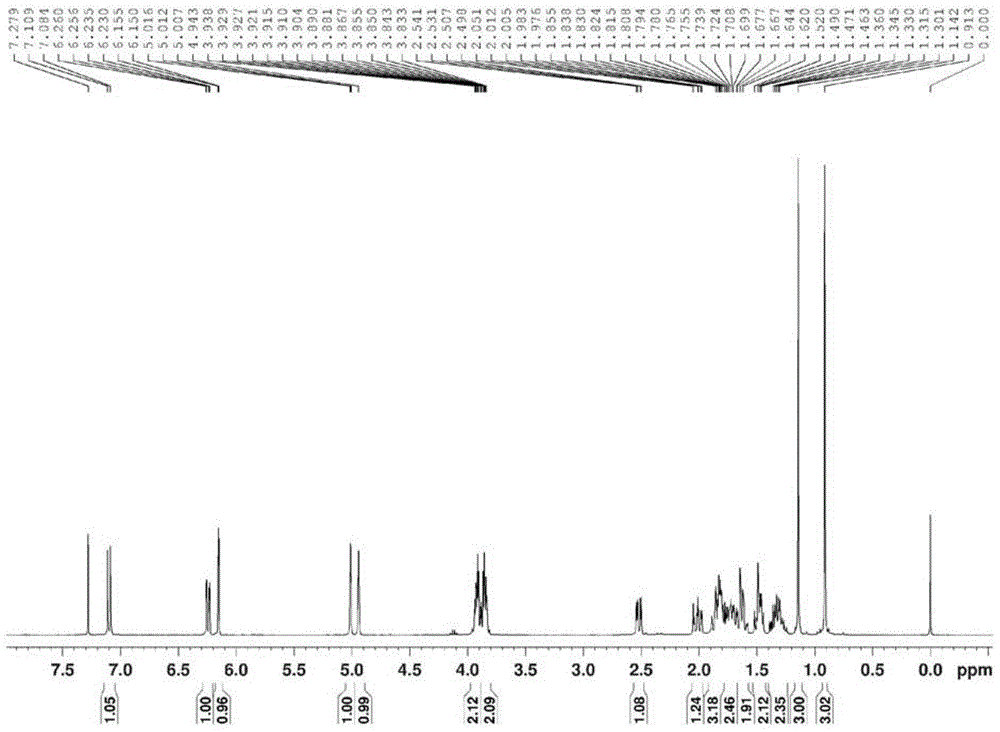
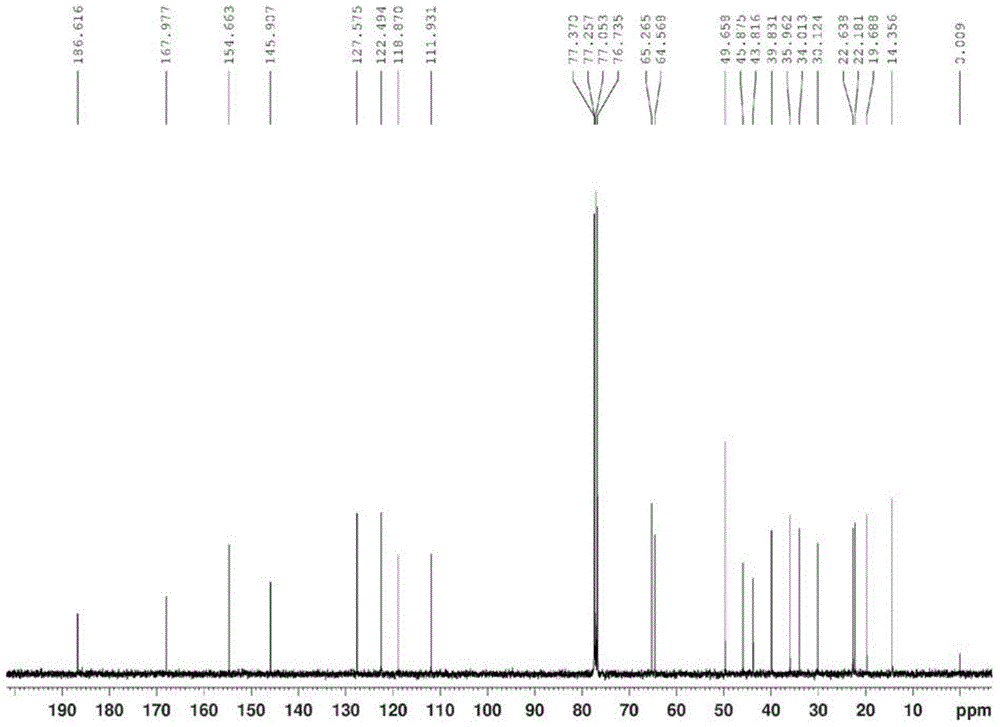
[0027] Product structure verification:
[0028] 1 HNMR (δ, ppm, 400MHz, CDCl 3 ): 7.062(d, J=10.0Hz, 1H, CH=CH); 6.315(d, J=10.0Hz, 1H, CH=CH); 6.070(s, 1H, CH=C); 3.817-3.925(m ,...
Embodiment 2
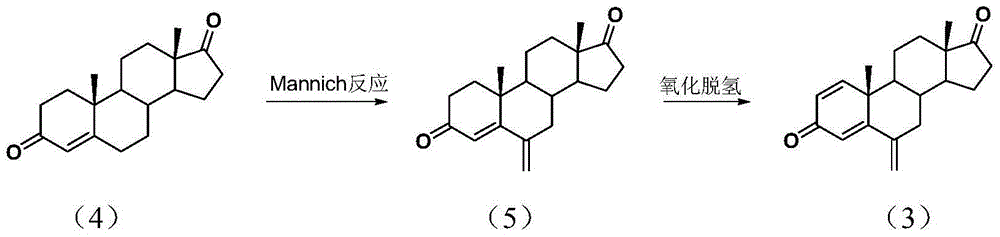
[0030] Embodiment 2: the preparation of 6-methylene androstienone ethylene glycol ketal (1)
[0031] Under the protection of nitrogen, 3.0 grams of paraformaldehyde (0.10mol) (according to formaldehyde conversion molar weight), 4.1 grams of dimethylamine hydrochloride (0.05mol) and 150 milliliters of isoamyl alcohol were added to 250 milliliters of the four-port reaction bottle, heated to reflux with water for 2 hours. Then add 3.28 grams of androstienone ethylene glycol ketal (0.01mol), insulate and stir the reaction at 140°C, track and detect the reaction by high-performance liquid chromatography, the reaction is completed in about 15 hours, cool to room temperature, add 100 milliliters of water to wash, and separate layers , after drying over anhydrous magnesium sulfate, the solvent was recovered under reduced pressure, and silica gel column chromatography (eluent: ethyl acetate:petroleum ether=4:6) gave 1.2 grams of white solid, liquid phase content 98.3%, yield 34.7%, mel...
Embodiment 3~8
[0036] Embodiment 3~8: Mannich reaction under different conditions prepares 6-methylene androstienone ethylene glycol ketal (1)
[0037]Under the protection of nitrogen, sequentially add paraformaldehyde, dimethylamine hydrochloride and 150 ml of isoamyl alcohol into a 250 ml four-necked reaction flask, and heat to reflux with water for 2 hours. Then add 3.28 grams of androstienone ethylene glycol ketal (0.01mol), stir the reaction at a certain temperature, track and detect the reaction by high performance liquid chromatography to the end of the reaction, cool to room temperature, add 100ml of water for washing, layering, anhydrous sulfuric acid After the magnesium was dried, the solvent was recovered under reduced pressure, silica gel column chromatography (eluent: ethyl acetate:petroleum ether=4:6), weighed, the liquid phase content was measured, and the yield was calculated. The results are shown in the following table.
[0038] Table 1: Prepared with different amounts of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com