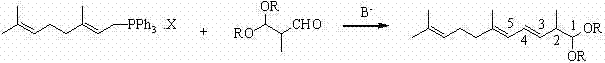
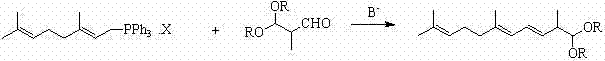
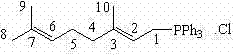Method for preparing 2,6,10-trimethyl-1,1-dialkoxyl-3,5,9-undecatriene
A technology of undecanetriene and dialkoxy, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult synthesis, difficult sources, difficult industrial production, etc., and achieve low cost , The process route is simple and the raw materials are easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of (2E)-3,7-dimethyl-2,6-octadiene-1-triphenylphosphonium chloride (3A).
[0038] In a 1000mL four-necked flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, add 90g of triphenylphosphine, 52g of geranyl chloride ((E)-1-chloro-3,7-dimethyl-2,6 -octadiene) and 600 ml of toluene, refluxed for 8 hours. Cool down, filter, and dry the solid at 60-70°C to obtain 117 g of a white solid, with a yield of 89.7%.
[0039] Product structure confirmation:
[0040]
[0041] 1 HNMR (400MHz, CDCl 3) δ (ppm): 1.383(d, J=3.6Hz, 3H, C-9H); 1.507(s, 3H, C-8H); 1.564(s, 3H, C-10H); 2.036(s, 4H, C-4H ,C-5H); 4.247(dd, J=15.6Hz, 7.6Hz, 2H, C-1H); 4.915-4.935(m, 1H, C-6H); 5.134-5.183(m, 1H, C -2H); 7.722-7.880(m, 15H, -(C 6 H* 5 ) 3 ).
[0042] 13 CNMR (100MHz, CDCl 3 ) δ(ppm):15.685, 15.713; 16.548; 22.346,22.848; 24.636; 25.439; 25.472; 39.182, 39.211; 108.302, 108.397; 118.118, 118.970; 123.455; 130.038, 130.274; 131.637; 133...
Embodiment 2
[0044] Example 2: Preparation of (3-E and 3-Z)-2,6,10-trimethyl-1,1-dimethoxy-3,5,9-undecanetriene (2A).
[0045] In a nitrogen-protected 500ml three-neck flask, add 43.5 g of (2E)-3,7-dimethyl-2,6-octadiene-1-triphenylphosphonium chloride (3A) (0.10 mol) and 100 Add 12.4 grams of potassium tert-butoxide (0.11 mol) in batches at -30 to -25°C under mechanical stirring for about half an hour. After the addition, continue to insulate and stir for about 1 hour to fully dissociate the carbanions. Then keep -30~-25℃ and add 13.2 grams of 2-methyl-3,3-dimethoxy-1-propanal (4A) (0.10 mol) dropwise, and the dropwise addition is completed in about 1 hour, and continue to keep stirring for about half After 1 hour, add 50 ml of water and 100 ml of diethyl ether and stir for 10 minutes, separate layers, evaporate the diethyl ether to dryness, add 100 ml of n-hexane and 100 ml of 1:1 methanol water (v:v) solution, stir for 10 minutes, then separate layers, n-hexane The alkane layer was wa...
Embodiment 3
[0052] Example 3: Preparation of tomato with (3-E and 3-Z)-2,6,10-trimethyl-1,1-dimethoxy-3,5,9-undecanetriene (2A) red pigment (1).
[0053] Under nitrogen protection, 12.6 grams of (3-E and 3-Z)-2,6,10-trimethyl-1,1-dimethoxy-3,5,9- Add undecanetriene (0.05mol), 100ml tetrahydrofuran and 1.2g p-toluenesulfonic acid into a 250ml four-neck flask, stir evenly, add 22g water dropwise, stir and react at 20-25°C for one day, and track the reaction by gas chromatography After the reaction was basically completed, a solution prepared by adding 2 grams of sodium bicarbonate and 20 milliliters of water was added for neutralization, and the tetrahydrofuran was distilled off under reduced pressure by a water pump, then 100 milliliters of cyclohexane was added, and the organic layer was separated after layering. The organic layer was washed with 30 ml of water, dried over anhydrous magnesium sulfate, and the solvent was recovered under reduced pressure to obtain 10.5 g of 2,6,10-trimeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com