Anti-human tissue transglutaminase 2 monoclonal antibody and application thereof
A technology of transglutaminase and antibody, applied in the direction of antibody, tissue culture, anti-enzyme immunoglobulin, etc., can solve the problems of poor tissue contact and hindering the therapeutic application of small molecule inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Polypeptide Design and Synthesis
[0043] Using literature search and protein amino acid sequence analysis software, the applicant found a highly conserved amino acid sequence in the enzymatic activity region of tissue transglutaminase 2 (tGM2), the sequence of which is shown in SEQ ID NO.1. This sequence is highly conserved among mice, rats and humans. The applicant carried out the synthesis of the polypeptide according to this sequence (synthesized by NeoBiolab, USA). Part of the polypeptide was coupled with the carrier protein Keyhole Limpet Hemocyanin (KLH) (synthesized by NeoBiolab, USA), and used as an immunogen to immunize rats.
Embodiment 2
[0044] Example 2: Preparation of anti-human tissue transglutaminase 2 (tGM2) monoclonal antibody
[0045] A. Immunization of SD rats
[0046] Three SD rats aged 12 weeks were immunized with KLH-polypeptide coupled protein as antigen. 250ug antigen was dissolved in sterile phosphate buffer and heated in a water bath at 50°C for 30 minutes, then mixed with Freund's complete adjuvant to make a homogeneous oil emulsion, and the antigen oil emulsion was injected into rats the abdominal cavity. After 3 weeks, the antigen was mixed with incomplete Freund's adjuvant by the same method and injected into the abdominal cavity of rats. After another 3 weeks, the antigen was mixed with Freund's incomplete adjuvant by the same method and injected into the abdominal cavity of rats. The immunization success of KLH-polypeptide coupled protein was determined by periocular blood sampling and enzyme-linked labeling methods 2 weeks after the last immunization.
[0047] B. cell fusion
[0048]...
Embodiment 3
[0056] Example 3: Identification of the functional activity of anti-human tGM2 monoclonal antibodies
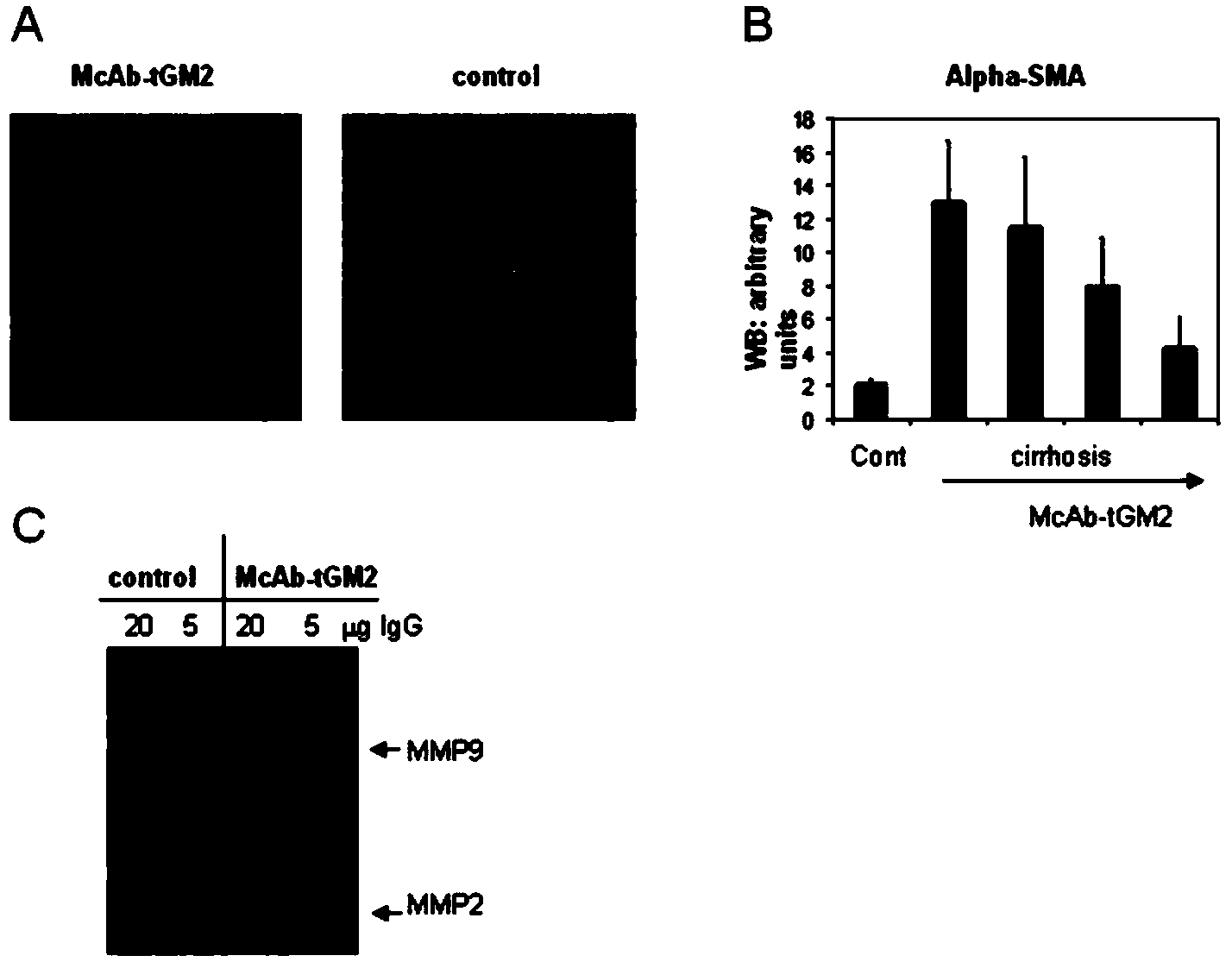
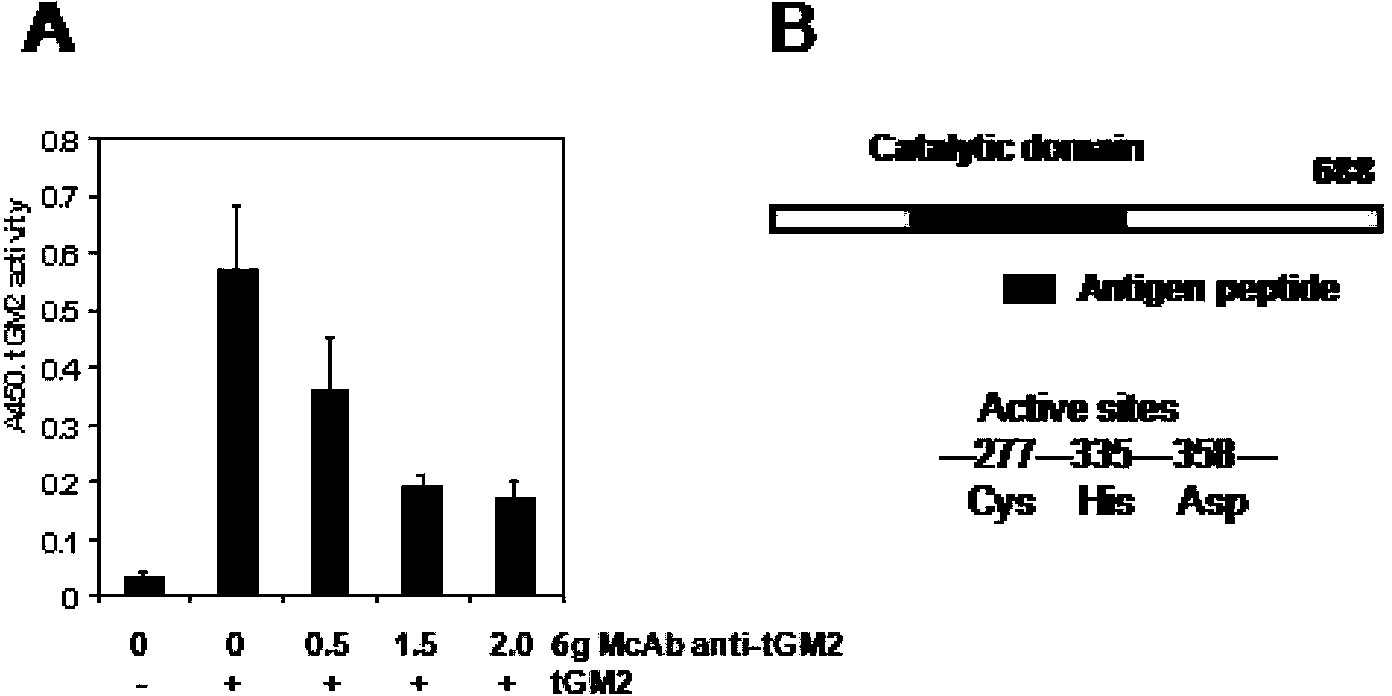
[0057] In order to detect the activity of monoclonal antibodies inhibiting tGM2, the applicant established an enzymatic reaction system. In this system, a peptide derived from human type I collagen was first modified with Biotin (Biotin-LSQQIENI), and then cross-linked to poly-lysine (poly-lysine) catalyzed by tGM2 ( image 3 ). The extent of the cross-linking reaction was monitored by avidin-HRP mediated enzymatic reaction. image 3 showed that the transamination activity of tGM2 was greatly inhibited as the concentration of anti-tGM2 antibody increased.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com