Preparation method of vitamin A acetate
A technology of acetate and vitamins, which is applied in the field of preparation of vitamin A acetate, can solve the problems of unfavorable environmental protection, harsh temperature requirements, and lower than -40°C, and achieve the effects of low cost, mild reaction conditions, and reduced output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
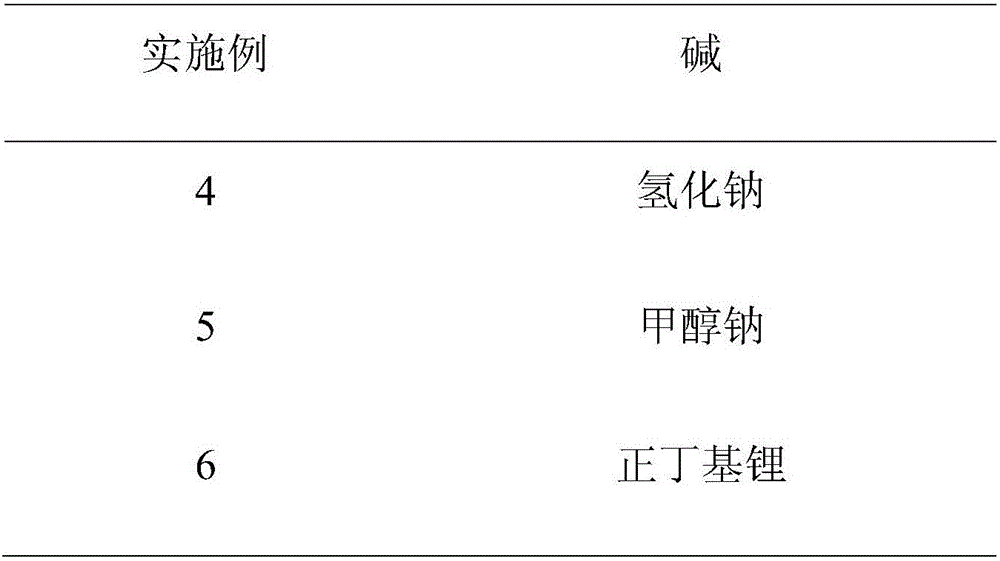
Embodiment 1
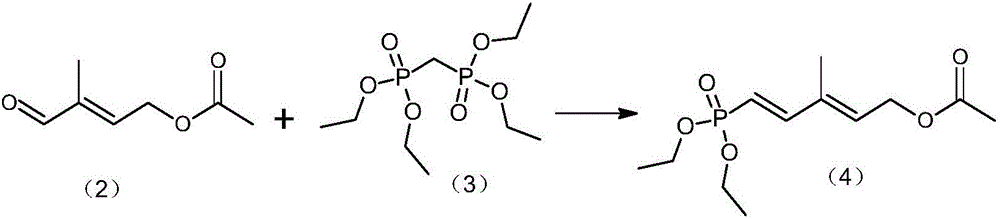
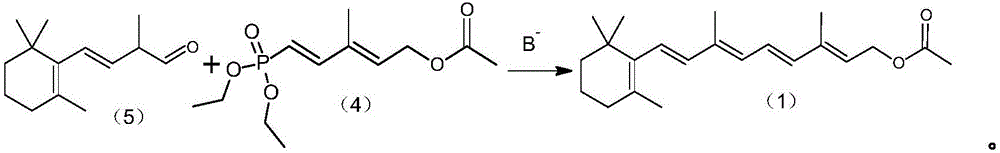
[0032] A preparation method of vitamin A acetate, comprising the following steps:
[0033] Step 1) Preparation of C6 phosphate (5-(diethyl phosphate)-3-methyl-2,4-hexadiene-1-acetate):
[0034] Under nitrogen protection, add 0.15mol sodium tert-butoxide and 50mL diethyl ether into a 500mL reaction flask, stir mechanically, cool down to about 0 °C, slowly add 0.10mol methylene diphosphate tetraethyl ester dropwise, control the internal The temperature does not exceed 0 °C, and the dropwise addition is completed in about 30 minutes. After the dropwise addition, the temperature is raised to 25-30 °C and the reaction is continued for 1.0 h. After completion of the reaction, 0.10mol of C5 aldehyde ester (3-methyl-4-oxo-2-butene-1-yl acetate) dissolved in 15mL ether solution was then added dropwise, and the internal temperature was controlled not to exceed 35 ℃, about 30 minutes, the dropwise addition was completed, and the reaction was continued at 20 ℃ for 2.0 h after the dropwis...
Embodiment 2
[0040] A preparation method of vitamin A acetate, comprising the following steps:
[0041] Step 1) Preparation of C6 Phosphate:
[0042] Under nitrogen protection, add 0.143mol butyllithium and 50mL dimethylformamide into a 500mL reaction flask, cool down to about 10°C with mechanical stirring, and slowly add 0.11mol tetraethyl methylene diphosphate dropwise. The internal temperature was controlled not to exceed 10°C, the dropwise addition was completed in about 30 minutes, and the temperature was raised to 25-30°C after the dropwise addition, and the reaction was continued for 1.0 h. The reaction was completed, then 0.1mol of C5 aldehyde ester dissolved in 10ml of dimethylformamide solution was added dropwise, the internal temperature was controlled not to exceed 5°C during the dropwise addition, the dropwise addition was completed in about 30 minutes, and the temperature was continued at 0°C after the dropwise addition. The next reaction was 2.0h.
[0043]After the reactio...
Embodiment 3
[0048] A preparation method of vitamin A acetate, comprising the following steps:
[0049] Step 1) Preparation of C6 Phosphate:
[0050] Under nitrogen protection, add 0.198mol sodium hydride and 75mL ethylene glycol dimethyl ether to a 500mL reaction flask, cool down to about 30°C with mechanical stirring, and slowly add 0.18mol tetraethyl methylene diphosphate dropwise. The inner temperature was controlled not to exceed 30°C, the dropwise addition was completed in about 30 minutes, and the temperature was raised to 25-30°C after the dropwise addition, and the reaction was continued for 1.0 h. After the reaction was completed, 0.15mol of C5 aldehyde ester dissolved in 15 mL of toluene solution was added dropwise. During the dropwise addition, the internal temperature was controlled not to exceed 35°C. The dropwise addition was completed in about 30 minutes. After the dropwise addition, the reaction was continued at 35°C for 2.0 hours.
[0051] After the reaction was complete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com