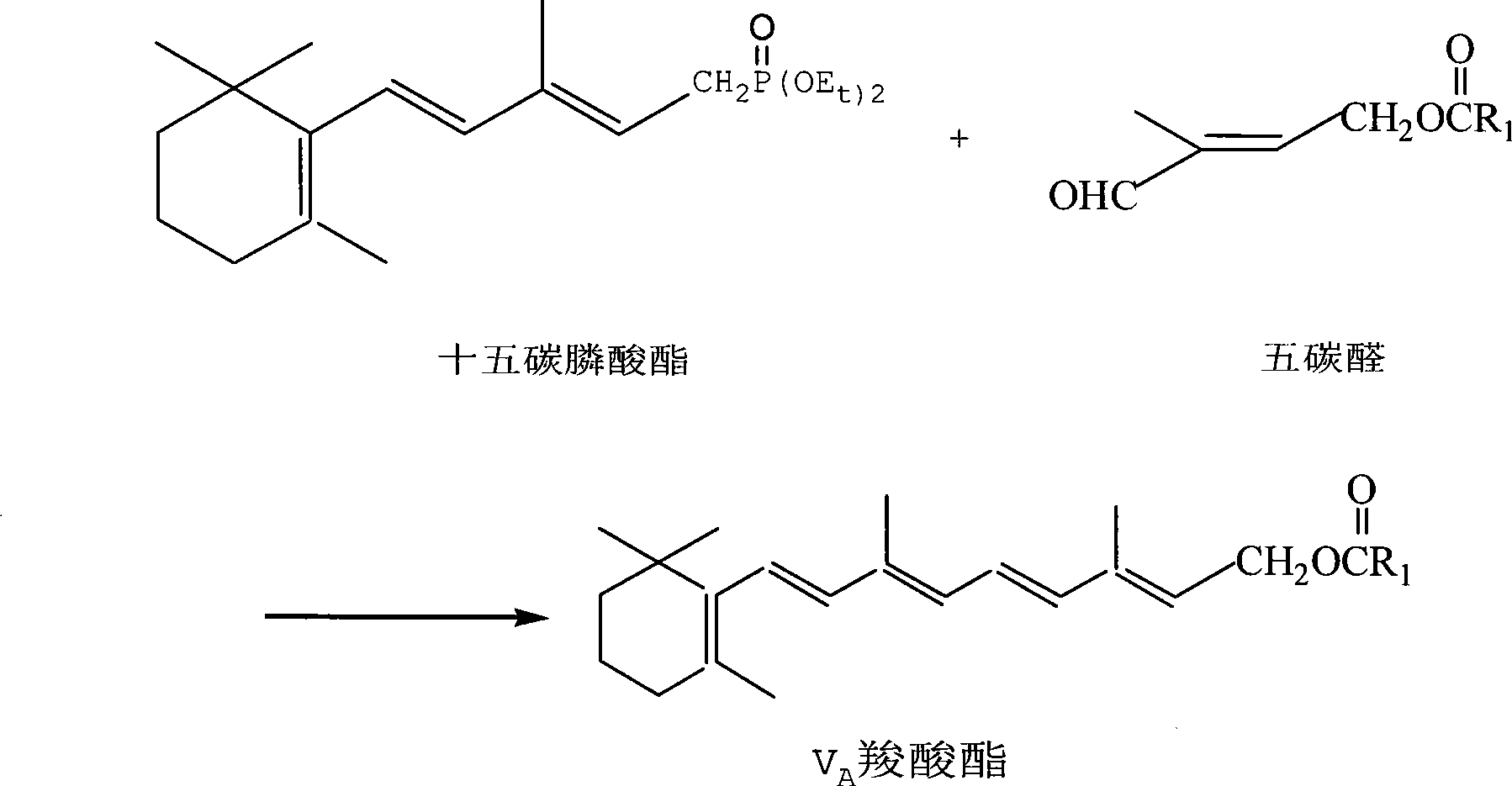
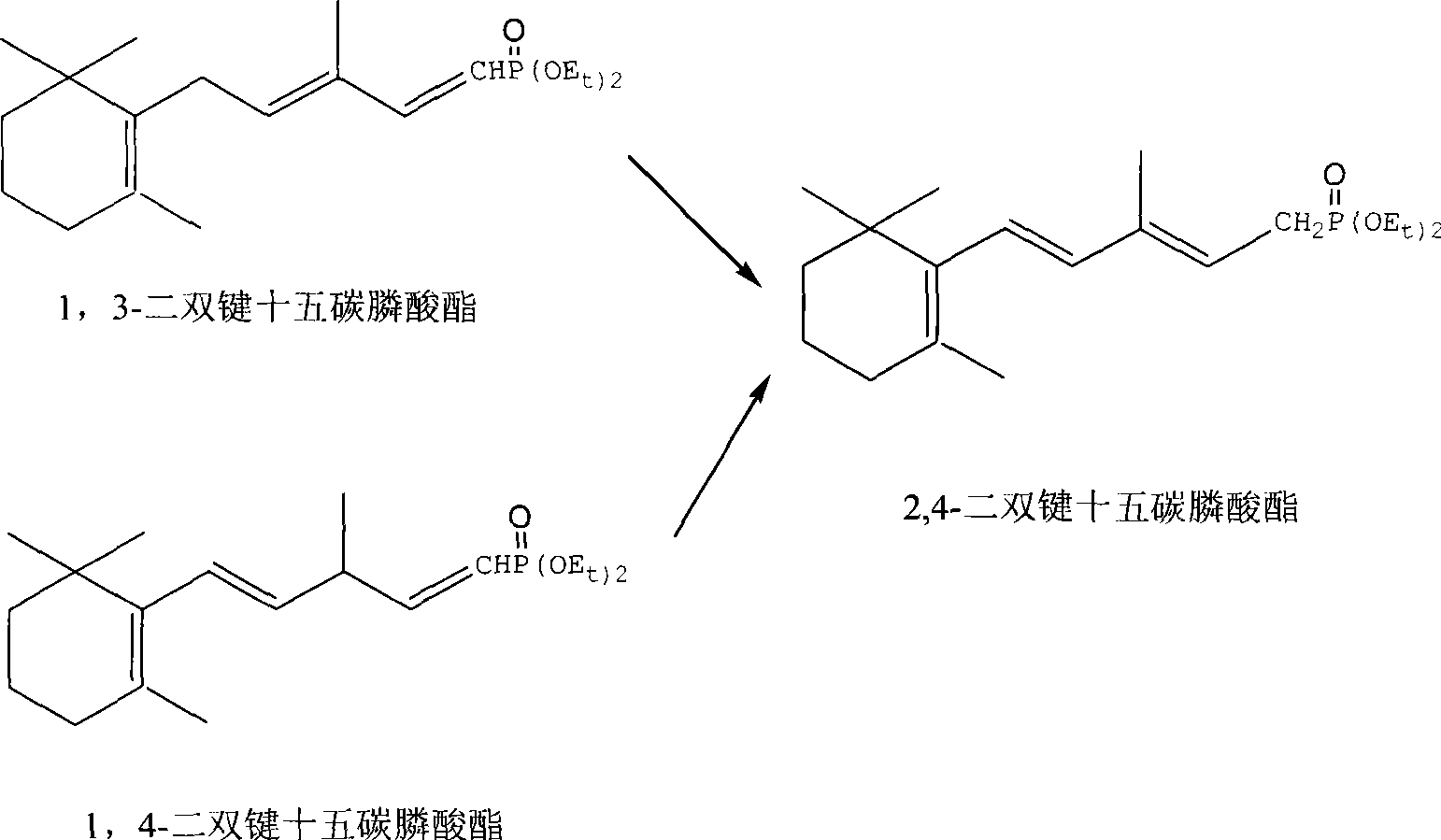
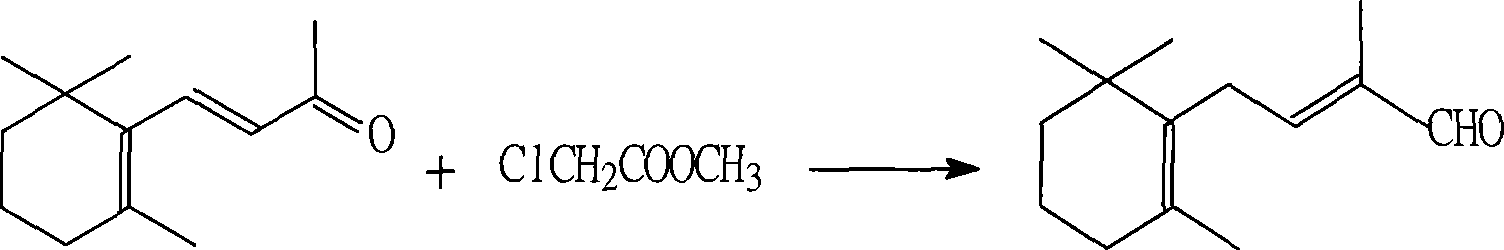Method for preparing 2,4-di-double-bond 15-carbon phosphonate
A technology of pentacarbon phosphonate and tetraethyl ethylene diphosphonate, which is applied in the field of 3-methyl-5--2, can solve the problem of long synthetic route and achieve the effect of simple and convenient process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of tetraethyl ethylene diphosphonate
[0039] In a 1000ml three-neck flask equipped with a thermometer and a reflux condenser; add 188g (1mol) of dibromoethane and 516g (3mol) of triethyl phosphinate, heat at 150-160°C for reflux reaction for 4 hours, and follow the reaction in the gas phase . Cool down to 80 DEG C afterwards, and the unreacted raw material is distilled off by water pump decompression; Yield 71%.
[0040] Structural validation:
[0041] 1 HNMR (δ, ppm, CDCl 3 ): 1.33 (t, J = 7.2Hz, 12H, -CH 3 ), 1.97-1.99 (m, 4H, -CH 2 -PO(OEt) 2 ), 4.06-4.179 (m, 8H, PO (O-CH 2 -) 2 )
[0042] 13 CNMR (δ, ppm, CDCl 3 ): 61.96 (O-CH 2 -), 19.16 (-CH 2 -P), 16.45(-CH 3 ) GC-MS (m / e): 302, 275, 257, 229, 201, 173, 165 (100%), 155, 137, 109
Embodiment 2
[0043] Embodiment 2: Preparation of 2,4-two double bond pentadecyl phosphonates
[0044] In a 500ml three-neck flask, add 30.2g of tetraethyl ethylene diphosphonate (0.1mol) and 100ml of ethylene glycol dimethyl ether, nitrogen protection, stirring and cooling to -35°C with a cold bath, and keep this temperature by adding 11.2g in batches Potassium tert-butoxide (0.1 mol) was added in about 10 minutes. After stirring for 10 minutes, a solution (0.1 mol) of 19.2 g of β-ionone dissolved in 100 ml of ether was added dropwise. The addition was completed in about half an hour, and then stirred for another half an hour. The TLC trace material basically disappeared (developing agent: ethyl acetate: petroleum ether = 1:3), left the cold bath, added 100ml of toluene and 100ml of water, and stirred for half an hour. The temperature was automatically raised to 12°C, the layers were separated, the aqueous layer was extracted with 100ml of toluene, the organic layer was combined, washed wi...
Embodiment 3
[0047] Embodiment 3: Preparation of 2,4-two double bond pentadecyl phosphonates
[0048] In a 500ml three-necked flask, add 30.2g of tetraethyl ethylene diphosphonate (0.1mol) and 100ml of tetrahydrofuran, stir and cool to -45°C with a cold bath, and add 10mol / L of butyl lithium dropwise with a syringe to maintain this temperature 10 ml (0.1 mol) of n-hexane solution was added in about 10 minutes. After stirring for 10 minutes, a solution (0.1 mol) of 19.2 g of β-ionone dissolved in 100 ml of tetrahydrofuran was added dropwise. The addition was completed in about half an hour, then stirred for another half an hour, and the TLC trace material basically disappeared (developer: ethyl acetate: petroleum ether = 1:3), added 100ml of toluene and 100ml of water, stirred for half an hour, during which the temperature rose automatically to 15°C, separate layers, extract the water layer with 100ml toluene, combine the organic layers, wash with 50ml water, and use the oil pump to distill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com