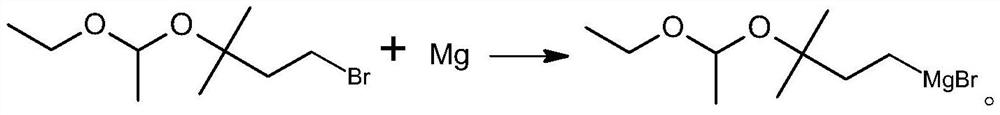Preparation method of C5 Grignard reagent
A technology of Grignard reagents and catalysts, which is applied in the field of preparation of C5 Grignard reagents, can solve problems such as difficult control of reactions, and achieve the effects of environmental protection, mild reaction conditions, and high light yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of C5-Grignard reagent, comprising the following steps:
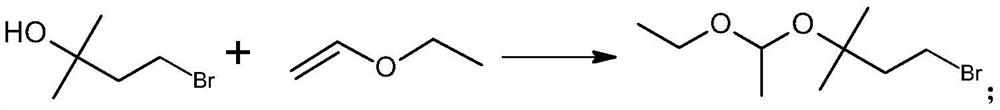
[0029] Step 1) Preparation of 1-bromo-3-(1-ethoxyethoxy)-3-methylbutane:
[0030] The reaction formula is:
[0031] The specific steps are: under the protection of nitrogen, add 0.10mol 2-methyl-2-butanol-4-bromine (C5 alcohol bromide) and 200mL anhydrous cyclohexane to a 500mL reaction flask, and cool down in an ice-water bath under magnetic stirring to Between 0 and 5°C, add 0.003mol p-toluenesulfonic acid monohydrate, stir to dissolve, first add 0.10mol vinyl ether at one time, and react for 1.0h between 0 and 10°C after adding, then add the remaining 0.10 at one time mol vinyl ether, after adding, continue to stir and react at 5-10°C, monitor the reaction process by GC, until the content of the raw material C5 alcohol bromide is not more than 1.0%, add 0.006mol triethylamine to terminate the reaction, continue stirring for 5min, add 10ml Stir in 1.0% sodium bicarbonate water for 10 mi...
Embodiment 2
[0038] A preparation method of C5-Grignard reagent, comprising the following steps:
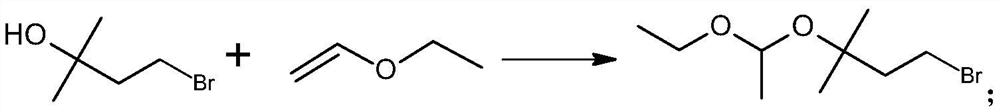
[0039] Step 1) Preparation of 1-bromo-3-(1-ethoxyethoxy)-3-methylbutane:
[0040] The reaction formula is:
[0041]The specific steps are: under the protection of nitrogen, add 0.10mol 2-methyl-2-butanol-4-bromine (C5 alcohol bromide) and 250mL anhydrous dichloromethane to a 500mL reaction flask, and cool down in an ice-water bath under magnetic stirring to Between 0~5℃, add 0.0035mol p-toluenesulfonic acid monohydrate, stir to dissolve, first add 0.11mol vinyl ether at one time, after adding, react at 0~10℃ for 1.0h, then add the remaining 0.11 mol vinyl ethyl ether, after adding, continue to stir and react at 5-10°C, monitor the reaction process by GC, until the content of the raw material C5 alcohol bromide is not more than 1.0%, add 0.007mol triethylamine to terminate the reaction, continue to stir for 5min, add 10ml Stir in 1.0% sodium bicarbonate water for 10 minutes for post-proces...
Embodiment 3
[0048] A preparation method of C5-Grignard reagent, comprising the following steps:
[0049] Step 1) Preparation of 1-bromo-3-(1-ethoxyethoxy)-3-methylbutane:
[0050] The reaction formula is:
[0051] The specific steps are: under the protection of nitrogen, add 0.10mol 2-methyl-2-butanol-4-bromine (C5 alcohol bromide) and 250mL anhydrous cyclohexane to a 500mL reaction flask, and cool down in an ice-water bath under magnetic stirring to 5°C, add 0.004mol p-toluenesulfonic acid monohydrate, stir to dissolve, first add 0.125mol vinyl ether at one time, after adding, react for 1.0h between 0-10°C, then add the remaining 0.125mol vinyl ether at one time After the addition, continue to stir the reaction at 5-10°C, monitor the reaction process by GC, until the content of the raw material C5 alcohol bromide is not more than 1.0%, add 0.008mol triethylamine to terminate the reaction, continue to stir for 5min, add 15ml 1.0% carbonic acid Sodium hydrogen water was stirred for 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com