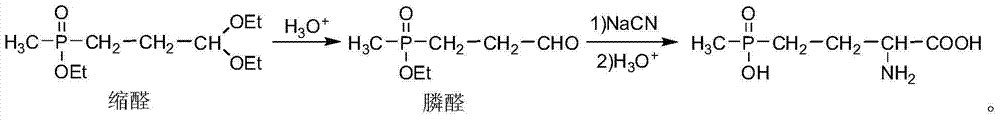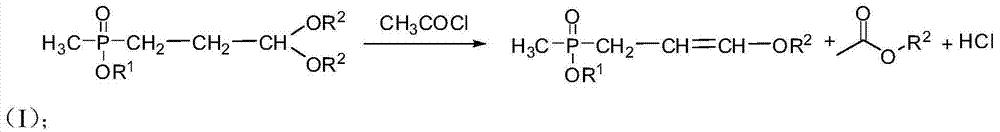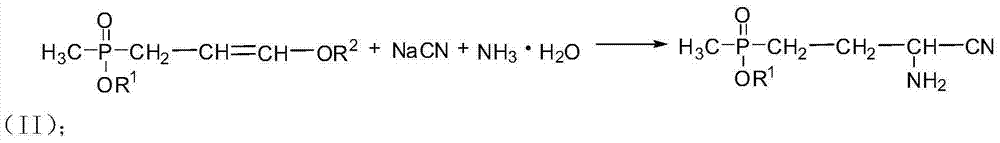Preparation method of amino-nitrile and intermediate for preparing glufosinate-ammonium
A technology of aminonitrile and alkyl, which is applied in the field of pesticide preparation, can solve the problems of low yield of glufosinate-ammonium, and achieve the effects of easy industrial production, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of ethyl 3,3-diethoxypropylmethylphosphonate
[0032] Add 143.2 g (1 mol) of diethyl methylphosphite with a content of 95% and 400 mL of absolute ethanol to a 1 L reaction flask, and add 57.7 g (1.02 mol) of acrolein with a content of 99% at a temperature of 20° C. dropwise under nitrogen protection. After the reaction at room temperature for 2 hours, the solvent and excess acrolein were recovered by distillation. The residue in the bottle was 243.5g, which was ethyl 3,3-diethoxypropylmethylphosphonate, with a content of 95% and a yield of 97.2%. .
[0033] (2) Preparation of ethyl 3-ethoxy allyl methyl phosphonate
[0034] At room temperature and in an inert gas protection atmosphere, 121.4g (0.5mol) of ethyl 3,3-diethoxypropylmethylphosphonate with a content of 98% was added to a 1L reaction flask, then 350g of tetrahydrofuran was added, cooled to 0°C, quickly add 41.2g (0.525mol) acetyl chloride dropwise, after the drop, the reactant was stirred at ...
Embodiment 2
[0038] (1) Preparation of methyl 3,3-dimethoxypropylmethylphosphonate
[0039] Add 170.5 g (1.5 mol) of dimethyl methylphosphite with a content of 95% and 500 mL of anhydrous methanol into a 1 L reaction flask, and add 87.7 g (1.55 mol) of acrolein with a content of 99% at 25°C under nitrogen protection, After dripping, react at room temperature for 2 hours, then distill to recover the solvent and excess acrolein. The residue in the bottle is 303.3g which is methyl 3,3-dimethoxypropylmethylphosphonate, with a content of 95% and a yield of 98% %.
[0040] (2) Preparation of 3-methoxyallyl methylphosphonic acid methyl ester
[0041] At room temperature and in an inert gas protection atmosphere, 303.3g (1.47mol) of 95% 3,3-dimethoxypropyl methylphosphonic acid methyl ester was added to a 1L reaction flask, cooled to -10°C, Quickly add 117g (1.49mol) of acetyl chloride dropwise. After the drop is completed, the reactant is stirred at room temperature for 2 hours, and the methyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com