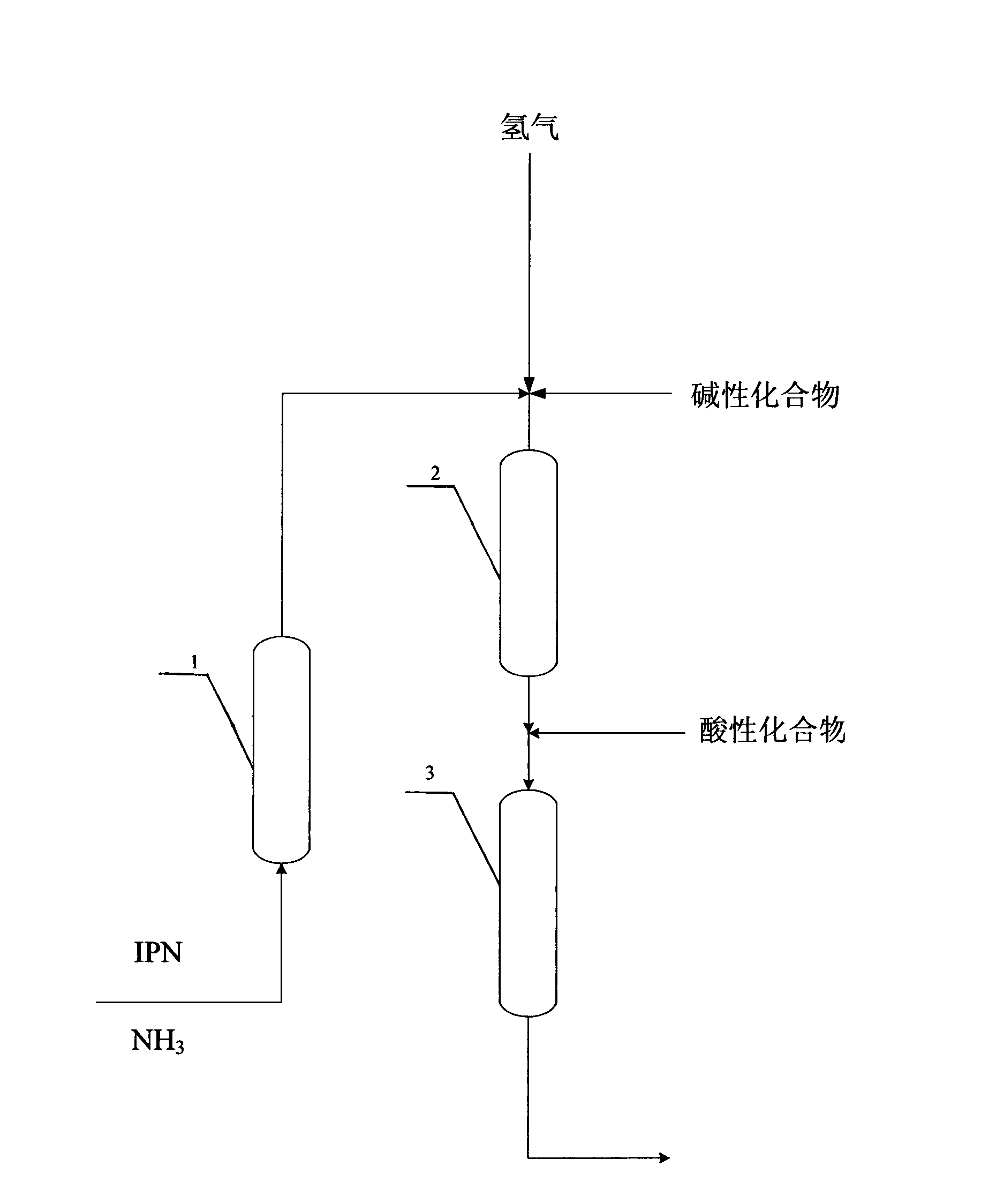
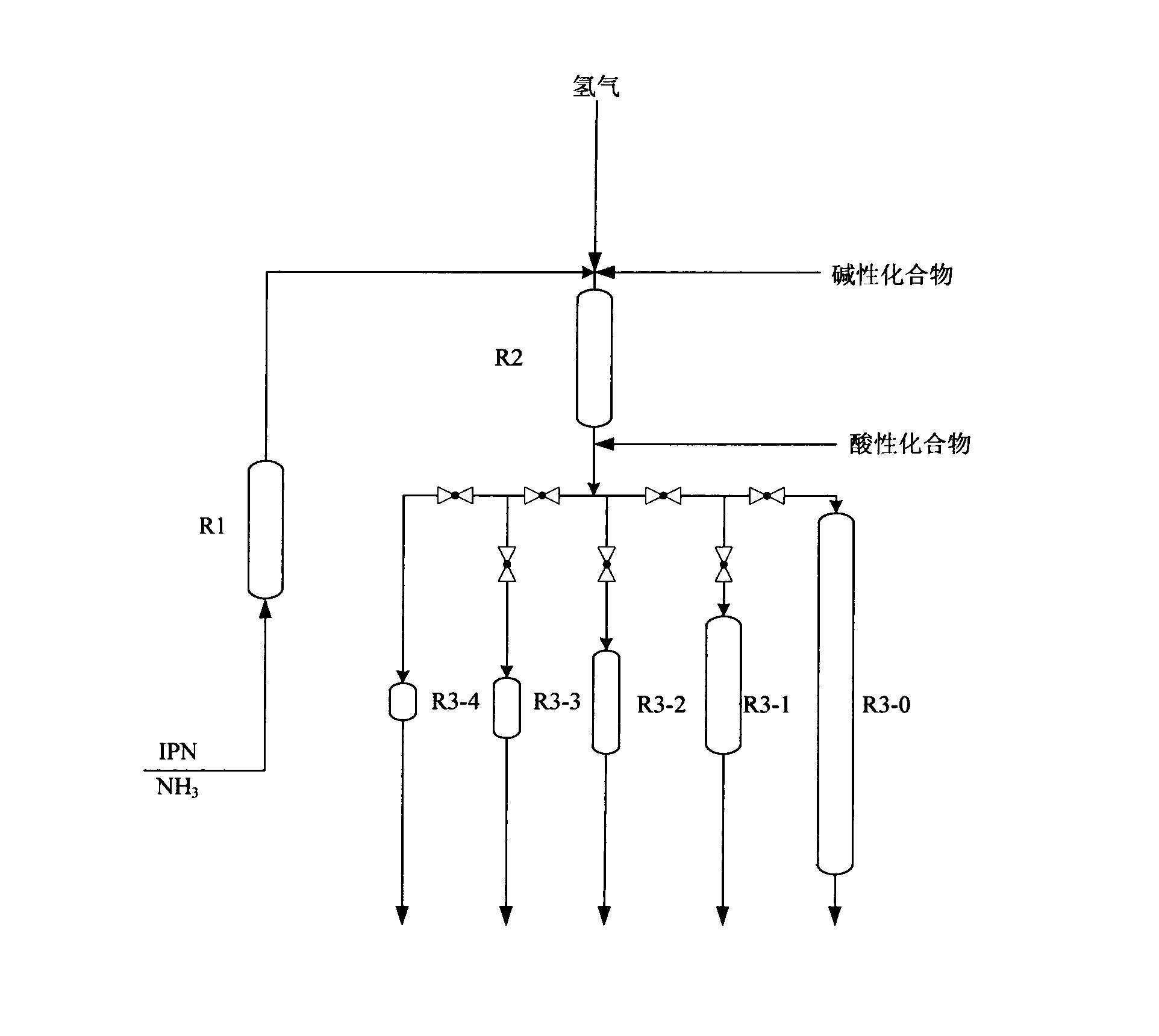
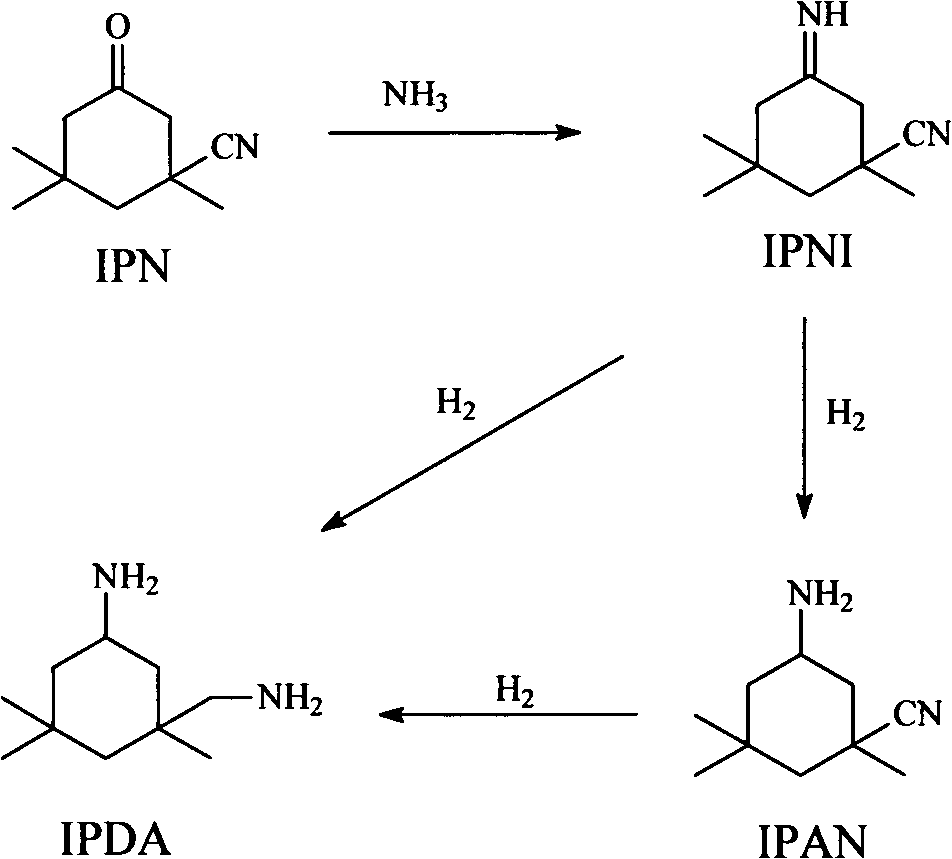
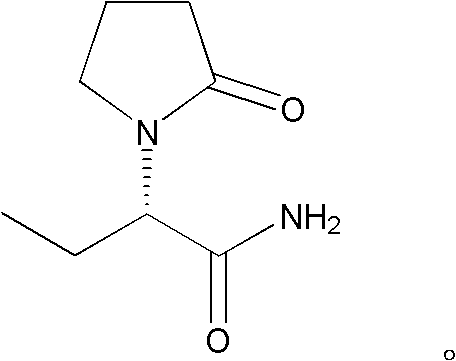
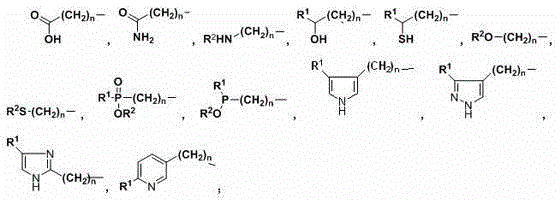
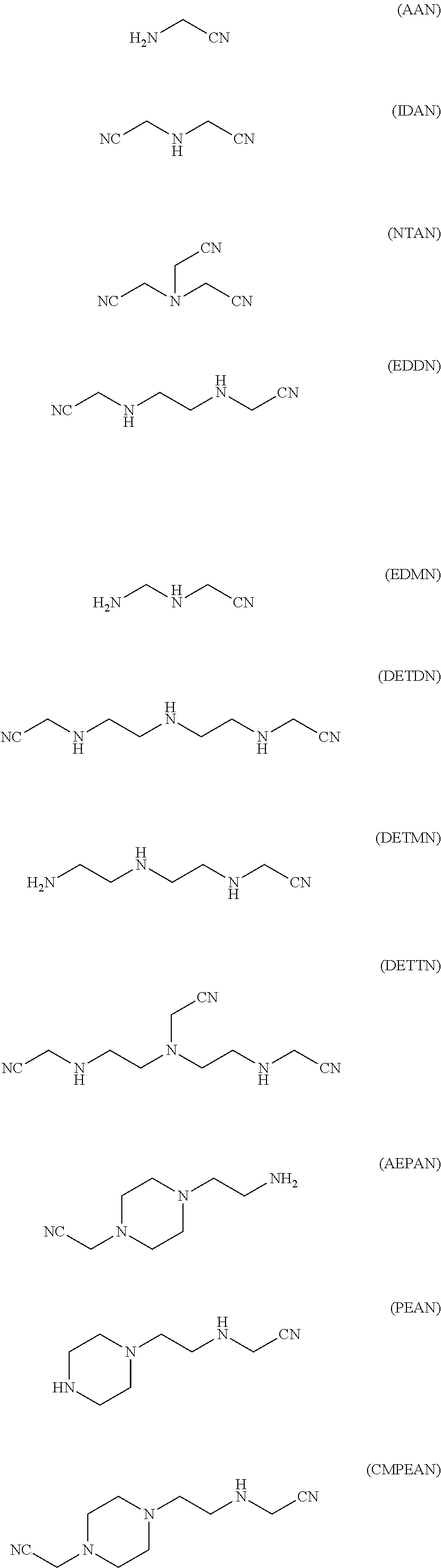
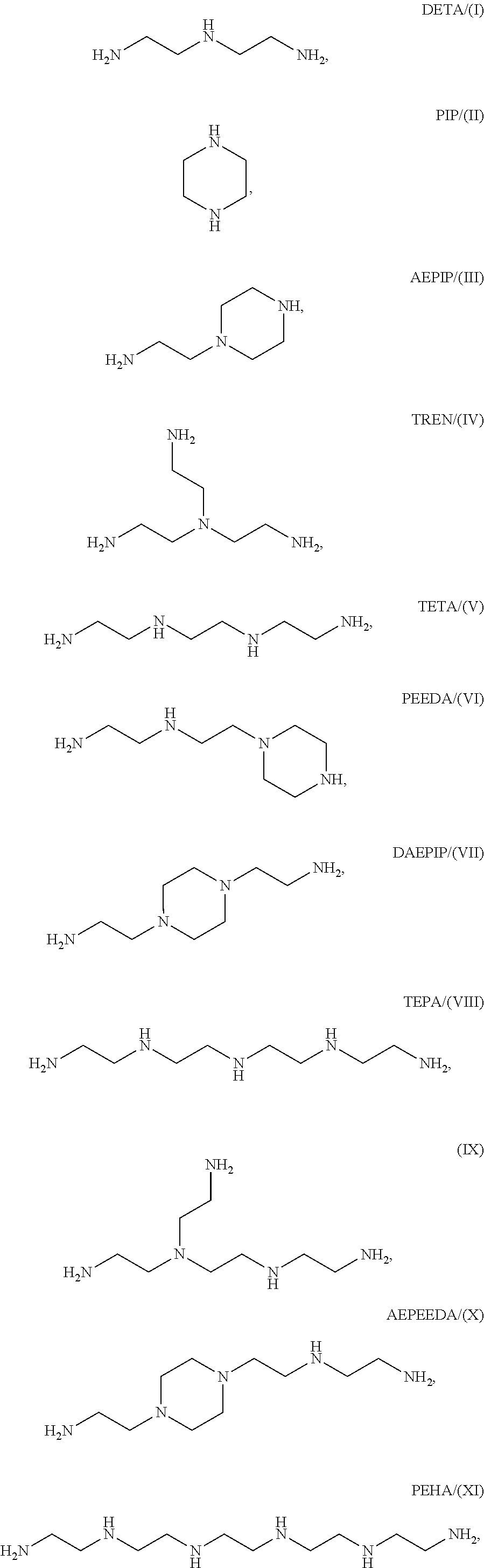
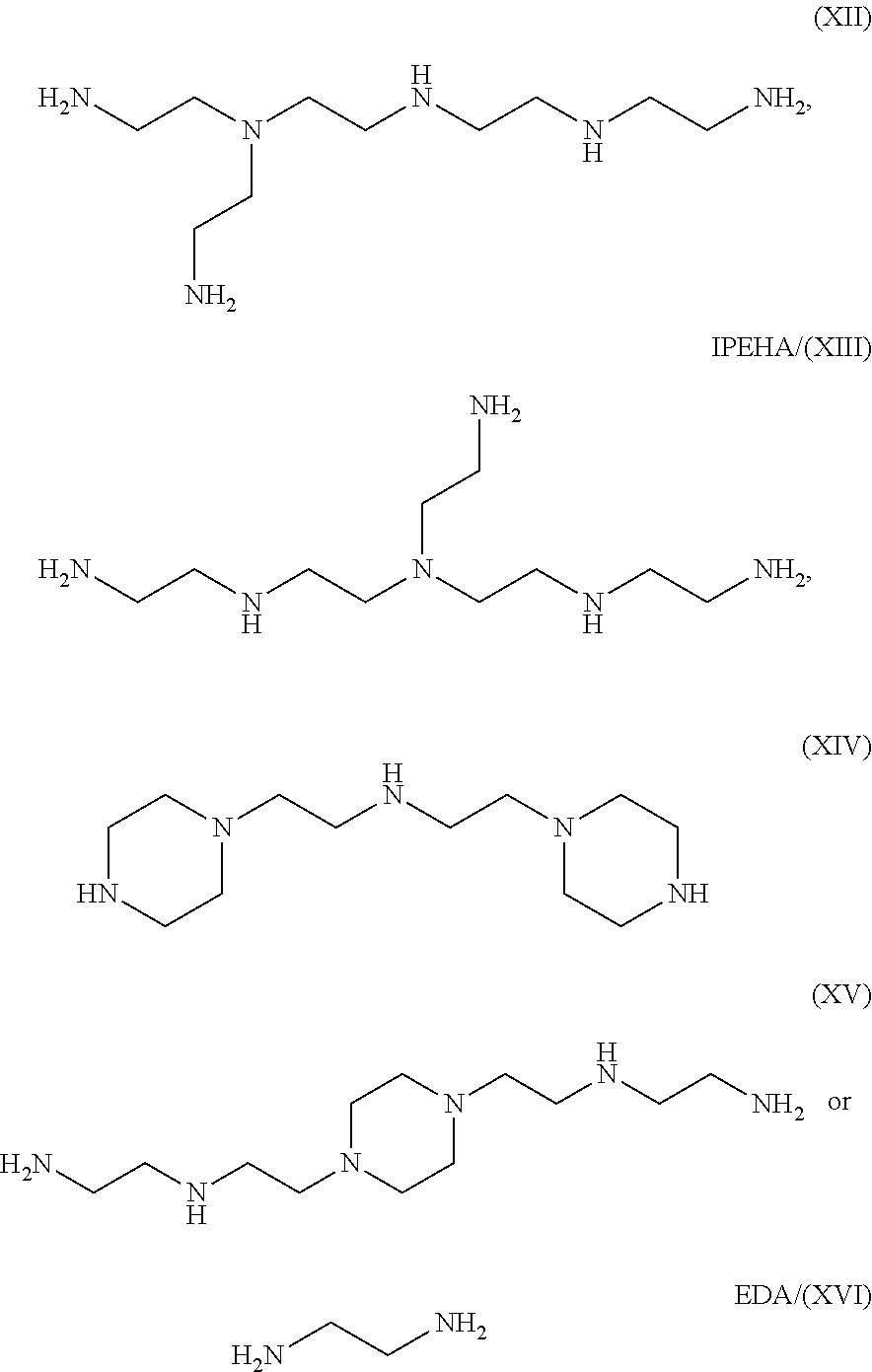
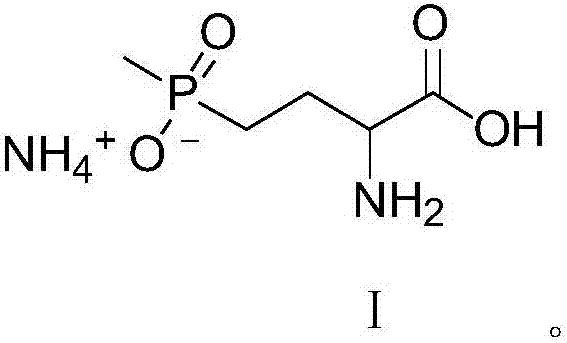
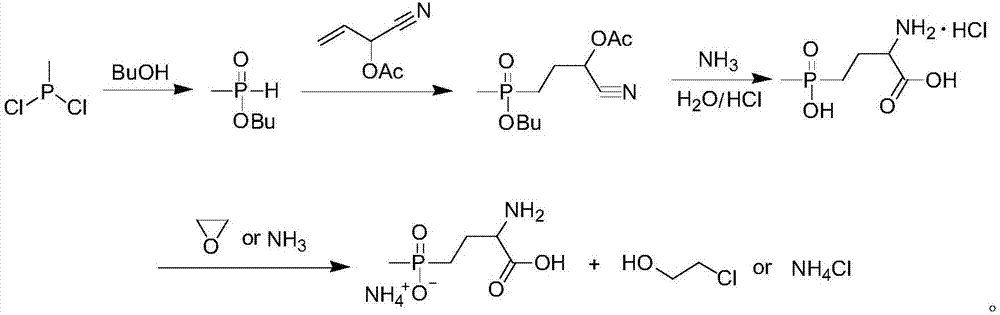
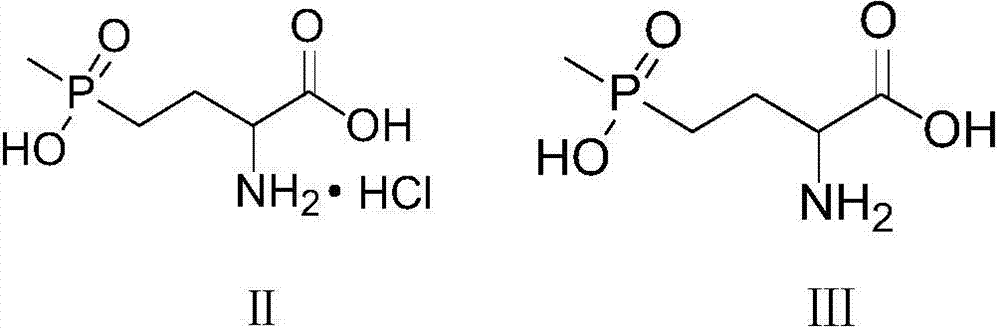
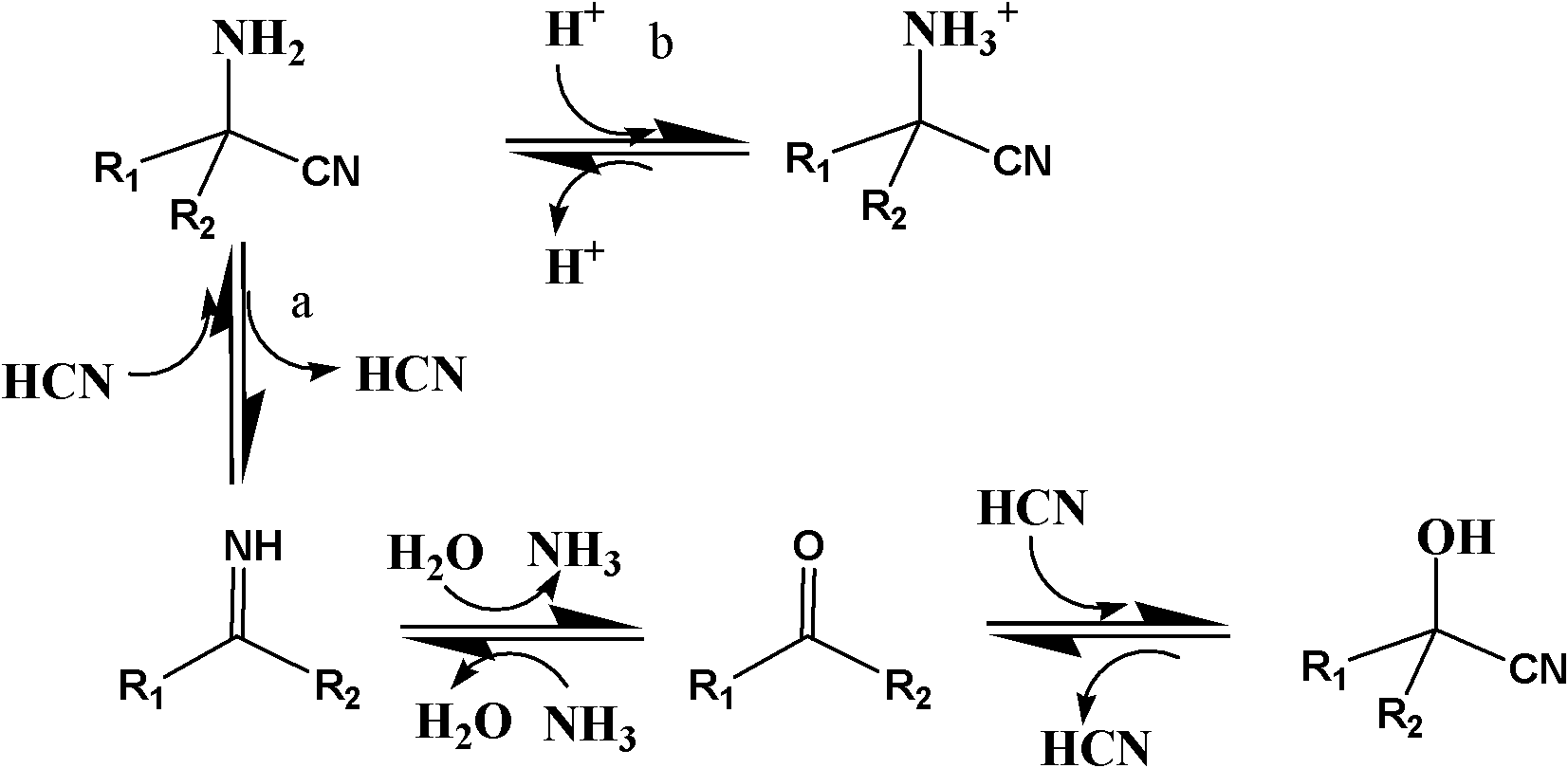
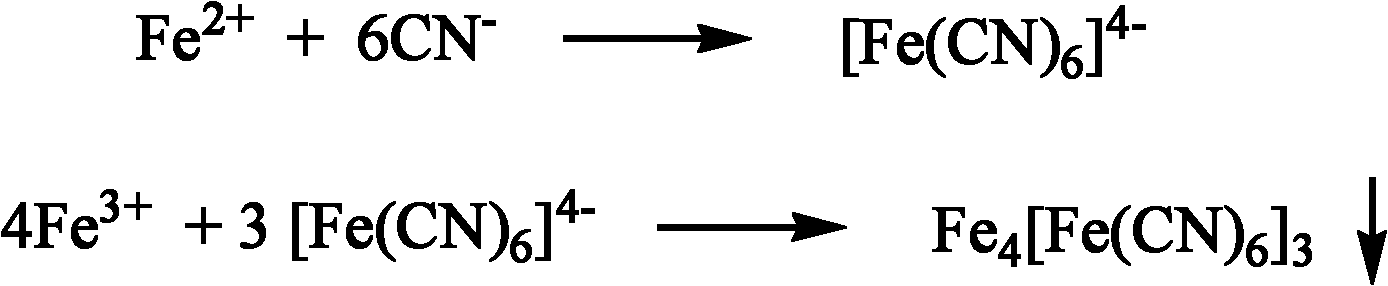
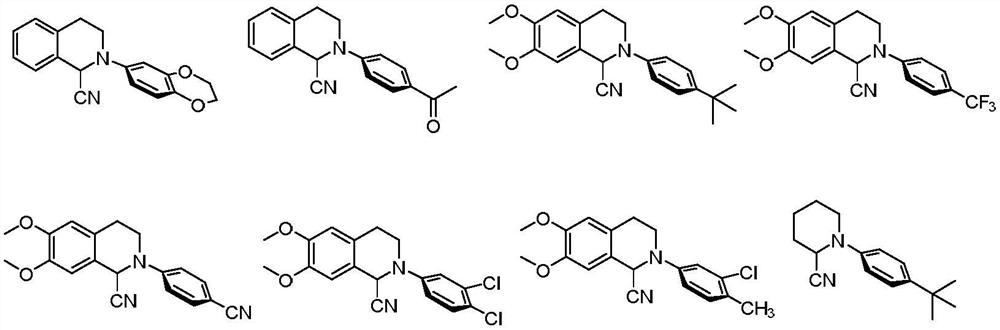
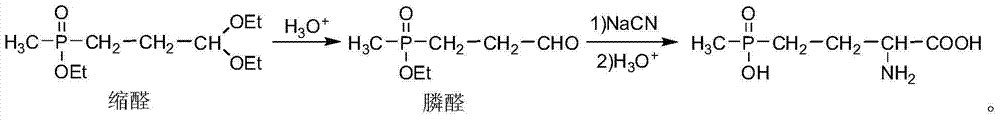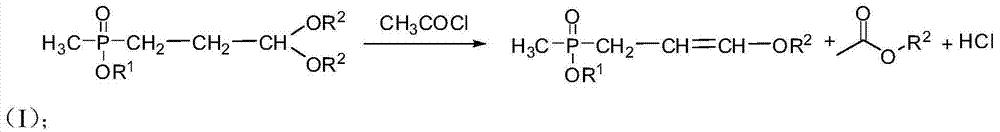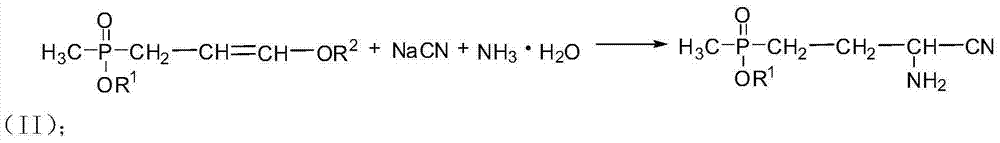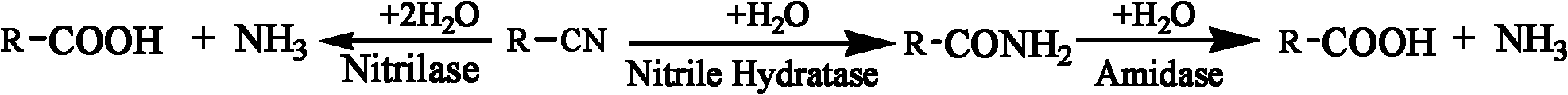Patents
Literature
89 results about "Amino nitriles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
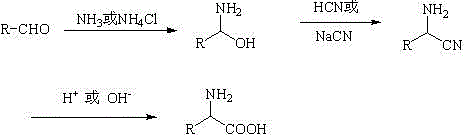
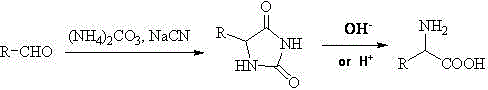
Amino Nitriles. Bifunctional building blocks are of special interest for drug design and organic synthesis due to three reasons, at least. First, these compounds can be used to tether two molecular fragments responsible for binding to the biological target, thus they can act as linkers. Second, if one functional group is not engaged in connection...
Process for producing polyamides from aminonitriles
A process for preparing a polyamide by reacting at least one aminonitrile with water comprises:(1) reacting at least one aminonitrile with water at a temperature from 100 to 360° C. and a pressure from 0.1 to 35x106 Pa to obtain a reaction mixture,(2) further reacting the reaction mixture at a temperature from 150 to 400° C. and a pressure which is lower than the pressure in step 1, the temperature and the pressure being selected so as to obtain a first gas phase and a first liquid or a first solid phase or a mixture of first solid and first liquid phase, and the first gas phase is separated from the first liquid or the first solid phase or from the mixture of first liquid and first solid phase, and(3) admixing the first liquid or the first solid phase or the mixture of first liquid and first solid phase with a gaseous or liquid phase comprising water at a temperature from 150 to 360° C. and a pressure from 0.1 to 30x106 Pa to obtain a product mixture.
Owner:BASF AG
Preparation method of 3-aminomethyl-3,5,5-trimethylcyclohexylamine
ActiveCN102924291AReduce dosageLess investmentCarboxylic acid nitrile preparationOrganic compound preparationHydrogenation reactionAmino nitriles
Owner:WANHUA CHEM GRP CO LTD +1
Method for regenerating powdery acticarbon
ActiveCN101590399AReduce dosageSolve the problem of energy consumptionOther chemical processesOrganic compound preparationActivated carbonHigh energy
The invention provides a novel method for activating and regenerating acticarbon and a novel process for decoloring. The invention utilizes the functions of oxidation and dehydration of sulphuric acid and carries out polymerization on organic pigment and modification on surface functional groups of the acticarbon to restore the adsorptive capacity of the acticarbon. The invention is in particular suitable for the field of preparation of amino acid through hydrolysis of amino nitrile, solves the difficult problems of difficult treatment of waste acticarbon, serious environmental pollution in the transferring and recycling process, high energy consumption and material consumption and poor applying effect, can achieve online recycling and use, can greatly reduce the use amount of the acticarbon and reclaim products entrained in the acticarbon; and the provided method is simple, is wide in application surface, clean and environment-friendly and has good environmental protection benefit and economic benefit.
Owner:BEIJING ZIGUANG YINGLI CHEM TECH CO LTD
A method for preparing (s)-2-aminobutanamide by enzymatic method
InactiveCN102260721AReduce processing costsCost advantageFermentationEnzymatic synthesisOrganic solvent
The invention discloses a synthesis process for preparing (S)-2-aminobutyramide by enzymatic method. The current preparation method has problems such as high cost, low yield, and large environmental hazards. The technical scheme adopted in the invention is: using 2-aminobutyronitrile as a starting material to prepare (S)-2-aminobutyramide in one step in a buffer solution system through enzyme catalysis. In the present invention, water is used as a solvent in the main process, without using a large amount of organic solvents harmful to the environment, the whole process cost is low, and it is beneficial to large-scale industrial preparation.
Owner:SYNCOZYMES SHANGHAI
Method for synthesizing quinazoline-4-(3H)-ketone
InactiveCN102584718ARaw materials are easy to getSimple processOrganic chemistryOrganic synthesisPotassium
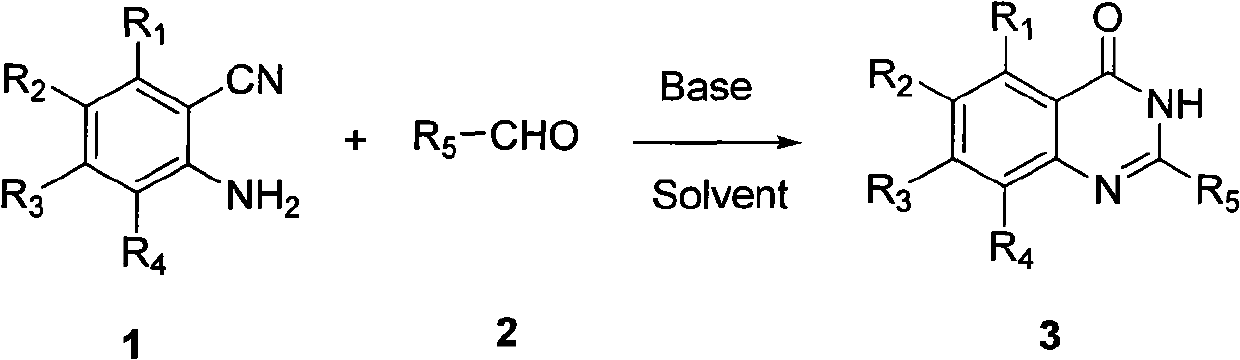
The invention provides a method for synthesizing quinazoline-4-(3H)-ketone and belongs to the field of organic synthesis. A reaction formula is expressed in a figure, wherein a reacting alkali is diethylamine, triethylamine, ammonium acetate, sodium (potassium) hydroxide and sodium (potassium) alkoxide; a reacting solvent is 1,4-dioxane, n-hexane, cyclohexane, tetrahydrofuran, benzene, toluene and dimethylbenzene; and the reaction implementation is conventional heating. According to the method, 2-amino-nitrile compound is taken as a raw material and is blended with aldehyde under the action of alkali, thereby synthesizing the quinazoline-4-(3H)-ketone in one step. The method has the advantages that the raw material is easily obtained, the process is simple, the reaction condition is mild, the reaction application scope is wide, the post-processing is simple and different substrates can be used for synthesizing the quinazoline-4-(3H)-ketone.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Preparation of aromatic nitrile compound by waste-free circulation method
InactiveCN101081821AReduce consumptionFull recoveryCarboxylic acid nitrile preparationOrganic compound preparationChemical industryCyclic process
The present invention is no-waste liquid cyclic process for preparing aromatic amino nitrile compound. The cyclic process synthesizes aromatic amino nitrile compound R1-NH-R2-CN by using aromatic amine compound R1-NH2 and hydroxyl alkyl nitrile compound HO-R2-CN as materials and in several combined cyclic systems, and has no waste liquid drainage. The cyclic process has full utilization of materials, saving in resource, high product yield, zero waste liquid drainage and other advantages, and is suitable for use in chemical industry.
Owner:成都圣洁环保有限责任公司
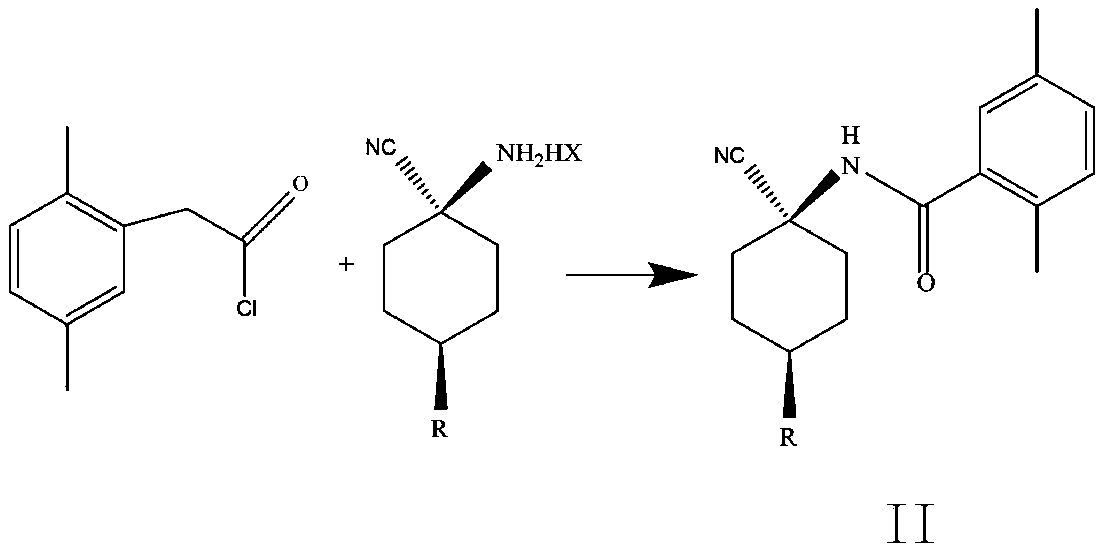
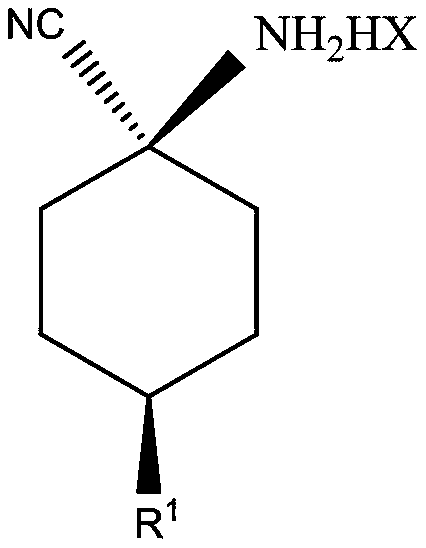
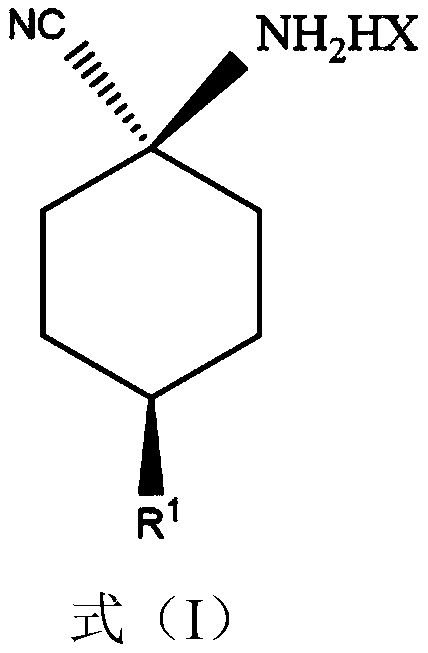
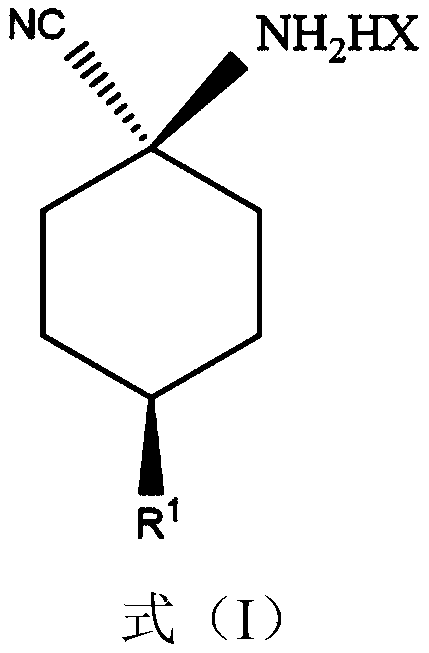
Cis-para-substituted cyclohexylamino nitrile salt and preparation method therefor
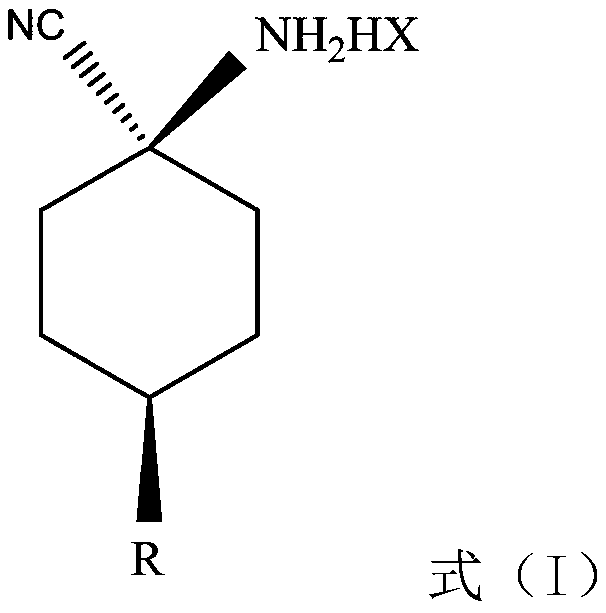
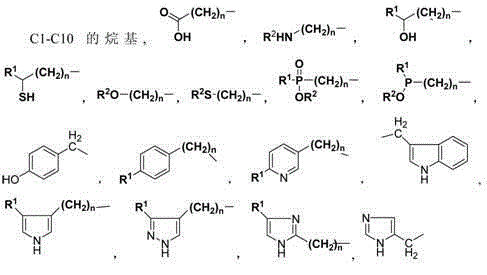
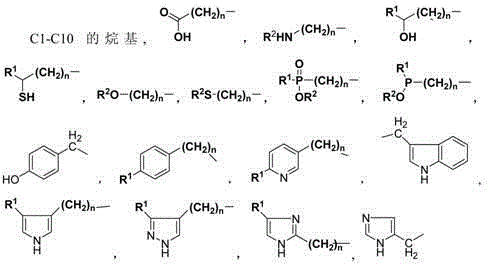
Provided are a cis-para-substituted cyclohexylamino nitrile salt as shown in formula (I) and a preparation method therefor, a use of said salt as a preparation intermediate for a pesticide such as spirotetramat, and a method for using said salt as an intermediate to prepare a pesticide such as spirotetramat. In formula (I), a para-substituent R is a C1-10 alkyl group or alkoxy group, a C1-10 alkenyl group or alkenyloxy group, a C1-10 alkynyl group or alkynyloxy group, a C1-10 cycloalkyl group or cycloalkoxy group, or a C1-10 heterocycloalkyl group or heterocycloalkoxy group containing 1-2 atoms selected from O and N, and HX is hydroxyacetic acid or maleic acid.
Owner:HEBEI LANSHENG BIOTECH CO LTD +1
Improved preparing method of cis-counterpoint substituted cyclohexyl amino nitrile maleate
The invention provides an improved preparing method of cis-counterpoint substituted cyclohexyl amino nitrile maleate shown in the formula (I). The method includes the steps of adding 0.3-0.55 molar fold of maleic acid to an organic solvent solution of a cis / trans-counterpoint substituted cyclohexyl amino nitrile compound corresponding to the formula (I) for stirring and ultrasonic treatment, and conducting filtering to obtain the cis-counterpoint substituted cyclohexyl amino nitrile maleate shown in the formula (I). In the formula (I), R1 represents a C1-C10 alkyl group or alkyl oxygroup, a C1-C10 vinyl group or vinyl oxygroup, a C1-C10 alkynyl group or alkynyl oxygroup, a C1-C10 naphthenic group or naphthenic oxygroup and a C1-C10 heterocyclic alkyl group or heterocyclic alkyl oxygroup with one or two heteroatoms selected from O and N, and HX represents maleic acid.
Owner:HEBEI LANSHENG BIOTECH CO LTD +2
Preparation method of amino-nitrile and intermediate for preparing glufosinate-ammonium
ActiveCN104497039AHigh yieldSatisfy productivityGroup 5/15 element organic compoundsGlufosinate-ammoniumSodium cyanide
The invention discloses a preparation method of amino-nitrile and an intermediate for preparing glufosinate-ammonium. The preparation method disclosed by the invention aims at solving the problem of low glufosinate-ammonium yield by using acetal in the existing methods. Different from the existing methods for preparing glufosinate-ammonium, the method disclosed by the invention comprises the following steps: firstly reacting acetal with acetylchloride to obtain an enol ether intermediate, reacting the enol ether intermediate with sodium cyanide to obtain amino-nitrile, and finally hydrolyzing the amino-nitrile to obtain the glufosinate-ammonium. The method has the advantages of higher reaction yield and capacity of remarkably reducing the production cost of the glufosinate-ammonium.
Owner:GUANGAN LIER CHEM CO LTD
Clean synthesis process of alpha-amino acid compounds
InactiveCN105037060AHigh purityProcess stabilityOrganic compound preparationGroup 5/15 element organic compoundsAlcoholDistillation
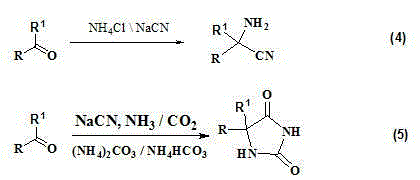
The invention relates to a clean synthesis process of alpha-amino acid compounds. The process specifically comprises the following steps of (1) adding one, two, or more of ammonia water / carbon dioxide, ammonium carbonate and ammonium bicarbonate into substituted alpha-amino nitrile or substituted hydantoin-based compounds in water or an alcohol-water mixture solvent, and carrying out heating for conducting a reaction; and (2) distilling the liquid to recover residual ammonium carbonate, ammonium bicarbonate or ammonia water after the reaction is finished, and enabling the distillation residue to crystallize in the alcohol solvent to obtain the alpha-amino acid compounds. The process provided by the invention is simple in operation, realizes recycling of materials, produces an extremely small amount of the three wastes, and is a truly clean process that should be vigorously promoted. The prepared alpha-amino acid compounds are high in yield and good in purity. The process is universally applicable to synthesis of multiple alpha-amino acid compounds.
Owner:HEBEI VEYONG BIO CHEM
Synthesis and purification method for alpha-amino acid compound
ActiveCN104892521AHigh purityReduce solubilityOrganic compound preparationGroup 5/15 element organic compoundsSolubilityPurification methods
The invention relates to a synthesis and purification method for an alpha-amino acid compound. The synthesis and purification method is characterized by comprising the following steps: (1) adding substituted alpha-amino nitrile or a substituted hydantoin-based compound into alkali M(OH)x or metal oxide MxO, adding water or an alcohol and water mixed solvent, and heating for reaction to obtain alpha-amino acid salt; (2) adding ammonium carbonate or ammonium bicarbonate or introducing carbon dioxide into the solution in the step (1), separating to obtain filter liquor and precipitates MxHyCO3, performing reduced pressure concentration on the filter liquor, and recrystallizing in an alcohol solvent to obtain the alpha-amino acid compound (I). The synthesis and purification method for the alpha-amino acid compound is simple, the yield and purity of the obtained alpha-amino acid compound are high; furthermore, recycling utilization and cleaning production of materials can be realized; the synthesis and purification method is especially suitable for synthesis of the alpha-amino acid compound with high water solubility.
Owner:HEBEI VEYONG BIO CHEM
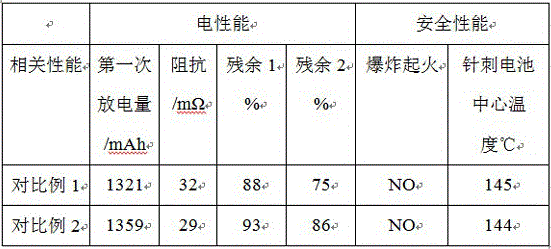
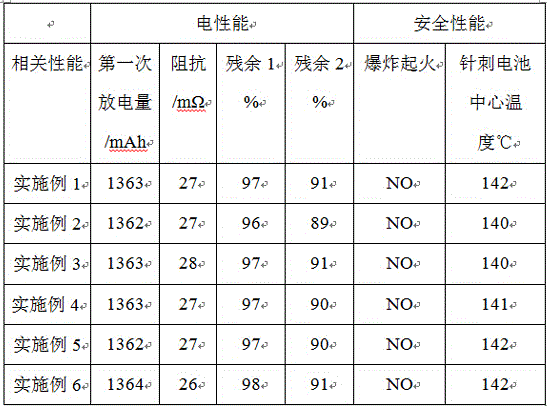
Positive electrode additive of lithium battery
ActiveCN105742706AExtended service lifeImprove cycle lifeSecondary cellsNickel–lithium batteryEngineering
The invention relates to a positive electrode additive of a lithium battery. The additive is modified maleimide modified by a combination containing amino-nitrile, annular alkali and annular acid; and after needle test on the battery prepared from the positive electrode additive, a result shows that the stability of the obtained positive electrode active material of the battery is greatly improved, and the cycle lifetime of the battery is prolonged. The positive electrode additive has the advantages that the used additive forms a protection film on the surface of the positive electrode active material, the positive electrode active material can be prevented from splitting during internal short circuit, and the battery is safer; and meanwhile, the good safe protection film is formed on the surface of the positive electrode active material, the service lifetime of the lithium battery is greatly prolonged, the cycle lifetime of the battery is also prolonged, and the self-discharging performance of the lithium battery is also greatly improved.
Owner:朱政
Production method for ethyleneamine mixtures
InactiveUS7880036B2Simple and inexpensiveSpeed up the conversion processCarboxylic acid nitrile preparationOrganic compound preparationSolventAmino nitriles
The invention relates to a process for preparing an ethylene amine mixture, which comprises hydrogenating an amino nitrile mixture comprising at least two α-amino nitriles in an amount of at least 5% by weight in each case in the presence of a catalyst and, if appropriate, a solvent.
Owner:BASF AG
Preparation method of glufosinate-ammonium acid
The invention discloses a preparation method of glufosinate-ammonium acid. The preparation method comprises the following steps: mixing glufosinate-ammonium amino nitrile with an alkali liquid for hydrolysis, and an absorbing produced ammonia gas with water; and adding oxalic acid into obtained hydrolysate for salt forming reaction; cooling a reaction liquid, filtering to obtain a filtrate and solid oxalate, concentrating the filtrate, adding alcohol into a concentrated liquid so as to separate solid glufosinate-ammonium acid, filtering, washing, and drying, so as to obtain the glufosinate-ammonium acid. According to the preparation method, glufosinate-ammonium amino nitrile is hydrolyzed by virtue of sodium hydroxide or calcium hydroxide and reacts with oxalic acid, ammonia generated through hydrolysis is deaminized through a micro-negative pressure and is absorbed with water so as to generate ammonia water, sodium oxalate or calcium oxalate is directly separated due to small water solubility or insoluble property, ammonium chloride is not generated in the whole preparation process, and the waste water quantity is extremely low. Prepared oxalate can be utilized for preparing oxalic acid again for circular use. The purity of prepared glufosinate-ammonium acid can reach above 96.5%, and the yield of prepared glufosinate-ammonium acid is more than or equal to 80%.
Owner:NANJING REDSUN BIOCHEM CO LTD +1
High-throughput screening method of nitrile invertase
InactiveCN101838681AOvercoming time-consuming and laboriousOvercoming demandsMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementWater bathsHigh-Throughput Screening Methods
The invention provides a high-throughput screening method of nitrile invertase, which comprises the following steps of: dissolving a sample to be tested in distilled water; adding a substrate namely an amino-nitrile compound, a hydroxy-nitrile compound or an amide compound into the mixture until the concentration of the substrate is 1 to 100 mM; performing a conversion reaction in a water bath at the temperature of between 10 and 50 DEG C for 10 to 120 minutes; taking the conversion liquid, adding Fe2<+> ion aqueous solution and Fe3<+> ion aqueous solution into the conversion liquid successively to perform a color reaction, wherein the quantity ratio of the added substrate to the Fe2<+> to the F3<+> is 1:1-3:1-3, the time interval for adding the two ions is 0 to 10 minutes; and judging the type of the contained nitrile invertase according to color changes of the solution. The complex color reaction-based high-throughput screening method of the nitrile invertase can better overcome the defects of the conventional screening method, can quickly identify whether the tested sample has the nitrile hydratase, amidase or nitrilase through simple color contrasts, has the advantages of simple and convenient operation, quick detection, economical efficiency, practicability and the like, and greatly facilitates the quick screening of the nitrile hydratase, the amidase or the nitrilase.
Owner:ZHEJIANG UNIV OF TECH
Aminonitrile production
InactiveUS6455723B1High selectivitySpeed up the conversion processCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystSelenium Compound
Owner:INVISTA NORTH AMERICA R L
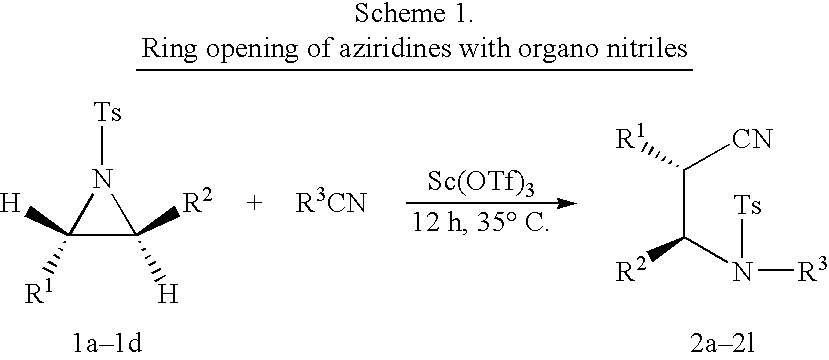
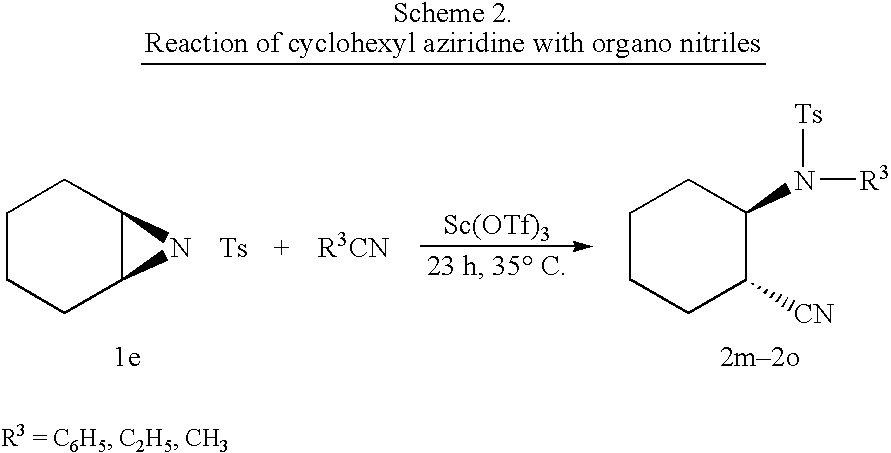
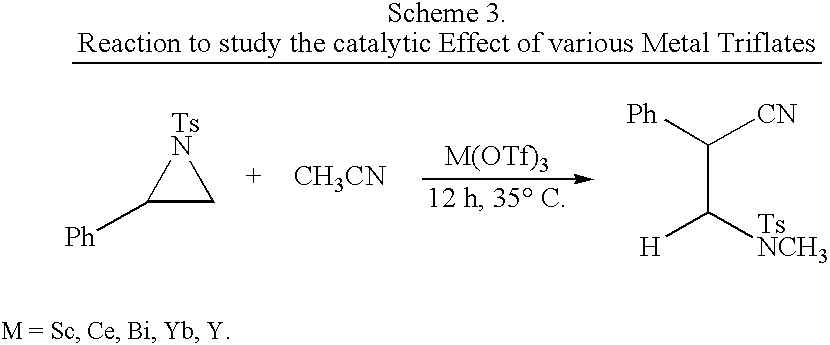
Process for the synthesis of N-substituted beta-amino nitriles through the ring opening of aziridines
InactiveUS20040198999A1High selectivityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationNitriteAziridine
The present invention comprises the simultaneous ring opening and concomitant N-substitution of various N-tosyl aziridines with different aliphatic and aromatic nitrites in presence of catalytic amount of metal triflates to afford different N-substituted beta-amino nitrites in excellent yields and selectivities.
Owner:COUNCIL OF SCI & IND RES
Preparation method of 2-aminobutanamide hydrochloride
InactiveCN101811978AMild reaction conditionsHigh yieldOrganic compound preparationCarboxylic acid amides preparationRoom temperatureSolvent
The invention discloses a preparation method of 2-aminobutanamide hydrochloride, which comprises the following steps that: ammonium chloride, ammonia (30 percent) and sodium cyanide are added into aqueous solvent, and propyl aldehyde is dripped in at 5 to 10DEG C to react for 4 to 10h and to be extracted; organic phase is combined and dried; hydrogen chloride gas is fed in at room temperature until the pH value is 3 to 4, filtered to prepare 2-amino nitrile hydrochloride; and the product is dissolved in isopropanol, the temperature goes up, the hydrogen chloride gas is fed in until the solution is saturated, reaction is carried out for 4 to 5h, the product is cooled to room temperature and filtered, thereby a target product is obtained. The preparation method of 2-aminobutanamide hydrochloride has the advantages of mild reaction conditions, high yield, few by-products and the like.
Owner:EAST CHINA NORMAL UNIV
Method for producing ethyleneamines
InactiveUS7880035B2Speed up the conversion processSimple and inexpensiveCarboxylic acid nitrile preparationOrganic compound preparationEthylenediamineDiethylenetriamine
The invention relates to a process for preparing an ethylene amine mixture, which comprises hydrogenating an amino nitrile mixture comprising at least 30% by weight of aminoacetonitrile (AAN) and at least 5% by weight of iminodiacetonitrile (IDAN) in the presence of a catalyst. Ethylenediamine (EDA) and / or diethylenetriamine (DETA) and, if appropriate, further ethylene amines can be isolated from the ethylene amine mixtures obtained.
Owner:BASF AG
Method for preparing organic diamine from organic amide and device thereof
ActiveCN113582853ALow impurity contentHigh purityOrganic compound preparationChemical recyclingPtru catalystHydrogenation reaction
The invention provides a method for preparing organic diamine from organic amide and a device thereof, and the method comprises the following steps: (1) mixing and gasifying ammonia gas and organic amide to obtain a gasified material; carrying out ammoniation reaction on the gasified material under the action of an ammoniation catalyst to obtain a first reaction material; (2) carrying out first refining and first adsorption on the first reaction material to obtain an aminonitrile organic matter; (3) performing a hydrogenation reaction on the aminonitrile organic matter and hydrogen under the action of a hydrogenation catalyst, and obtaining a reacted material containing organic diamine; and (4) sequentially carrying out secondary refining and secondary adsorption on the reacted material to obtain an organic diamine product. According to the method, the organic amide is converted into the aminonitrile organic matter through the gas phase method, the aminonitrile organic matter is purified and then converted into the organic diamine through hydrogenation, the process is simple and easy to control, the yield and selectivity of the final organic diamine can be remarkably improved, and the economic benefits of the whole process are improved.
Owner:JIANGSU YANGNONG CHEM GROUP
Aminonitrile production
InactiveUS20030065209A1High selectivitySpeed up the conversion processCarboxylic acid nitrile preparationOrganic compound preparationHydrogenation processSolvent
Provided is a selective hydrogenation process for producing aminonitriles by contacting the corresponding dinitriles with a hydrogen-containing fluid in the presence of a hydrogenation catalyst, a solvent and an amide additive.
Owner:INVISTA NORTH AMERICA R L
Method for preparing alpha-aminonitrile and product and application thereof
ActiveCN112390696AHigh yieldHigh reactivityCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystAmino nitriles
Owner:NANJING AGRICULTURAL UNIVERSITY
Process for hydrogenating dinitriles in aminonitriles
InactiveCN1359369AHigh yieldHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationSolventHydrogenation process
Provided is a selective hydrogenation process for producing aminonitriles by contacting the corresponding dinitriles with a hydrogen-containing fluid in the presence of a hydrogenation catalyst, a solvent and an additive for improving the yield of and / or selectivity to the aminonitrile.
Owner:INVISTA TECHNOLOG IES S A R L
Clean production process for amino acids such as iminodiacetic acid
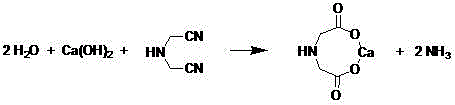
ActiveCN105985251AAvoid concentrationAvoid the Salt DifficultyOrganic compound preparationAmino-carboxyl compound preparationAlkaline hydrolysisAmino nitriles
The invention relates to a clean production method for amino acids such as iminodiacetic acid. The method comprises the steps: carrying out alkaline hydrolysis on amino-nitrile by adopting cheap lime cream, and carrying out deamination, so as to obtain an iminodiacetic acid calcium salt crude product; suspending the obtained iminodiacetic acid calcium salt crude product into water or mother liquor, adding sulfuric acid or other inorganic acid into the water or mother liquor for acidification, and filtering out an inorganic calcium salt filter cake with low water solubility; and subjecting the filter liquor to cooling and crystallization, carrying out separation so as to obtain iminodiacetic acid solids, and applying the crystallization mother liquor to an acidification reaction of next batch mechanically. During the acidification by the sulfuric acid, gypsum can be converted into calcium carbonate and ammonium sulfate by further using a byproduct, i.e., ammonia water of an alkaline hydrolysis process and carbon dioxide in flue gas, so as to directly generate compound fertilizers; the calcium carbonate can also be separated, and ammonium sulfate crystals can also be concentrated; and the calcium carbonate can be sold as a byproduct or be subjected to heated decomposition so as to obtain a quicklime raw material which can be applied mechanically. According to the novel process, the complete utilization of resources can be realized, the reaction selectivity is higher, the production of colored byproducts is reduced, and a concentrating process for removing waste salts such as sodium sulfate and sodium chloride and a wastewater treatment link are avoided; and due to concentration, the use level of a decolorant can be greatly lowered, the energy saving and consumption lowering effects are remarkable, and the production cost is reduced remarkably.
Owner:BEIJING ZIGUANG YINGLI CHEM TECH CO LTD
Aminonitrile production
InactiveUS6506927B1High selectivitySpeed up the conversion processCarboxylic acid nitrile preparationOrganic compound preparationHydrogenation processCyanate compound
Provided is a selective hydrogenation process for producing aminonitriles by contacting the corresponding dinitriles with a hydrogen-containing fluid in the presence of a hydrogenation catalyst, a solvent and a quaternary ammonium cyanate additive.
Owner:INVISTA NORTH AMERICA R L
Method for preparing aminonitrile organic matter by gas phase method
ActiveCN113582876AImprove conversion rateHigh selectivityOrganic compound preparationChemical recyclingPtru catalystOrganic matter
The invention relates to a method for preparing an aminonitrile organic matter by a gas phase method, a graded reaction mode is adopted in the method, the hot-spot temperature rise of the catalyst is reduced, the service life of the catalyst is prolonged, and the selectivity and conversion rate of the reaction are improved; in addition, the method adopts a purification mode combining refining and adsorption, so that the purity of the 6-aminonitrile organic matter product is also remarkably improved on the basis of saving energy consumption, and the influence of impurities on downstream products is reduced.
Owner:JIANGSU YANGNONG CHEM GROUP
Preparation method of alpha-amino-nitrile compound taking pyrrolidine tertiary amine as primer
InactiveCN109134338AIncrease productionLow pricePhysical/chemical process catalystsOrganic chemistrySolventPotassium ferrocyanide
The invention provides a preparation method of an alpha-amino-nitrile compound taking pyrrolidine tertiary amine as a primer. The preparation method comprises the following step of green synthesis ofalpha-amino-nitrile through a one-pot process by taking potassium ferrocyanide as a cyaniding reagent and an iodine phenyl compound as a reaction accelerant under the condition of a compound solvent containing trifluoroethanol and a catalyst. The invention provides the safer cyaniding reagent and an efficient synthetic method of alpha-amino-nitrile with mild reaction conditions. The preparation method is simple in post treatment, mild in reaction condition and short in reaction time, and develops a simple and efficient synthetic route for cyaniding synthesis of pyrrolidine tertiary amine.
Owner:求秋平
Method for synthesizing N-fatty acyl group amino acid
InactiveCN105348133ACarboxylic acid nitrile preparationOrganic compound preparationAmino nitrilesAmino acid
The invention discloses a method for synthesizing N-fatty acyl group amino acid. With fatty acid ester and amino-nitrile adopted as the raw materials, and with sodium methylate or sodium ethoxide adopted as the catalyst, the method comprises the following steps: heating under nitrogen protection; adjusting the pH value to be acid; after refrigeration, filtering the reaction liquid; carrying out reduced pressure distillation on the obtained solid; collecting the cut fraction, and placing in alkali liquor for hydrolysis, so as to obtain N-fatty acyl group amino acid salt; or collecting the cut fraction, and carrying out acidification to prepare N-fatty acyl group amino acid. According to the method, amino-nitrile and fatty acid ester are adopted as the raw materials to prepare high purity N-fatty acyl group amino acid, the raw materials are cheap, the equipment is simple, and waste water generated during the production process is less.
Owner:上海利盛生化有限公司
Preparation of chiral amino-nitriles
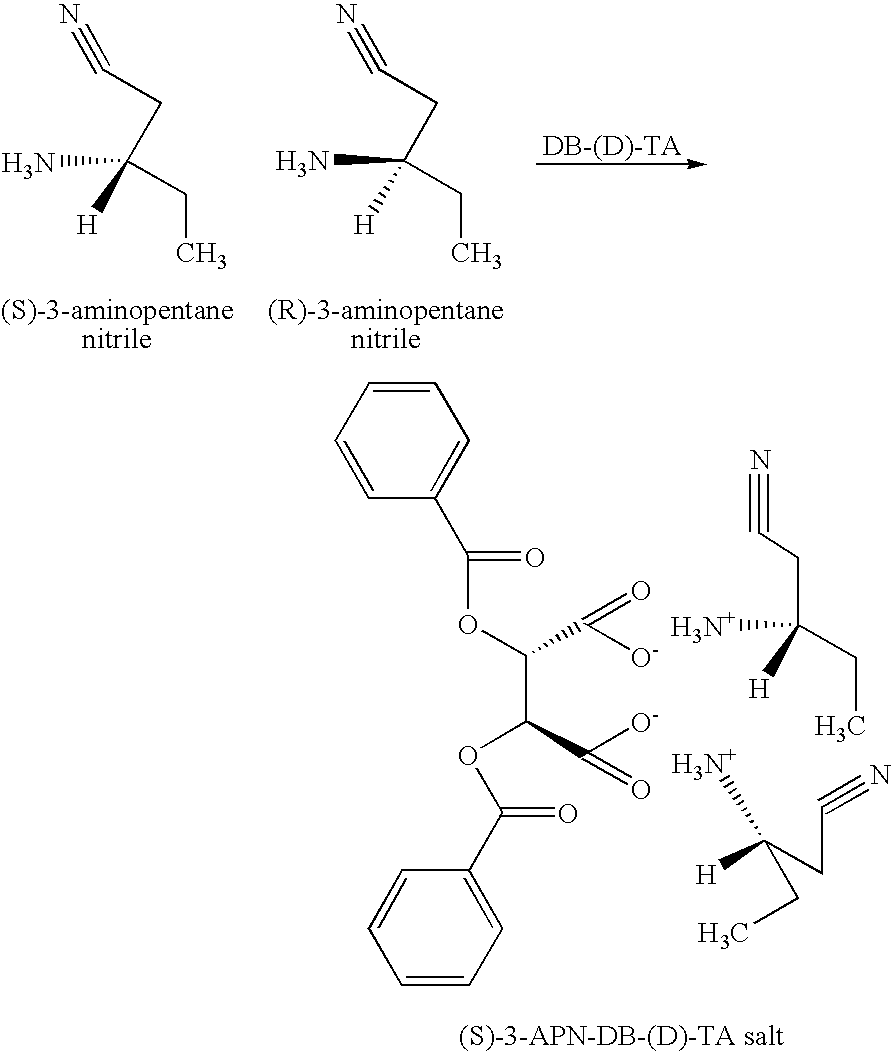
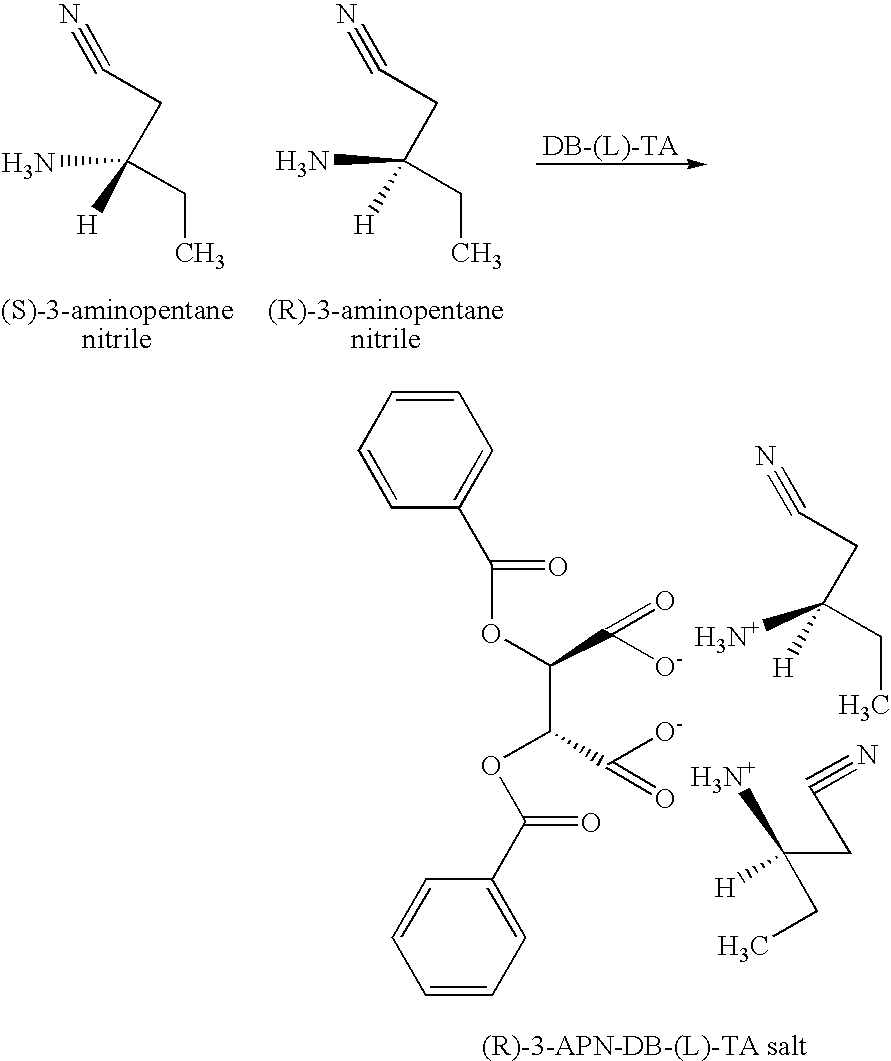
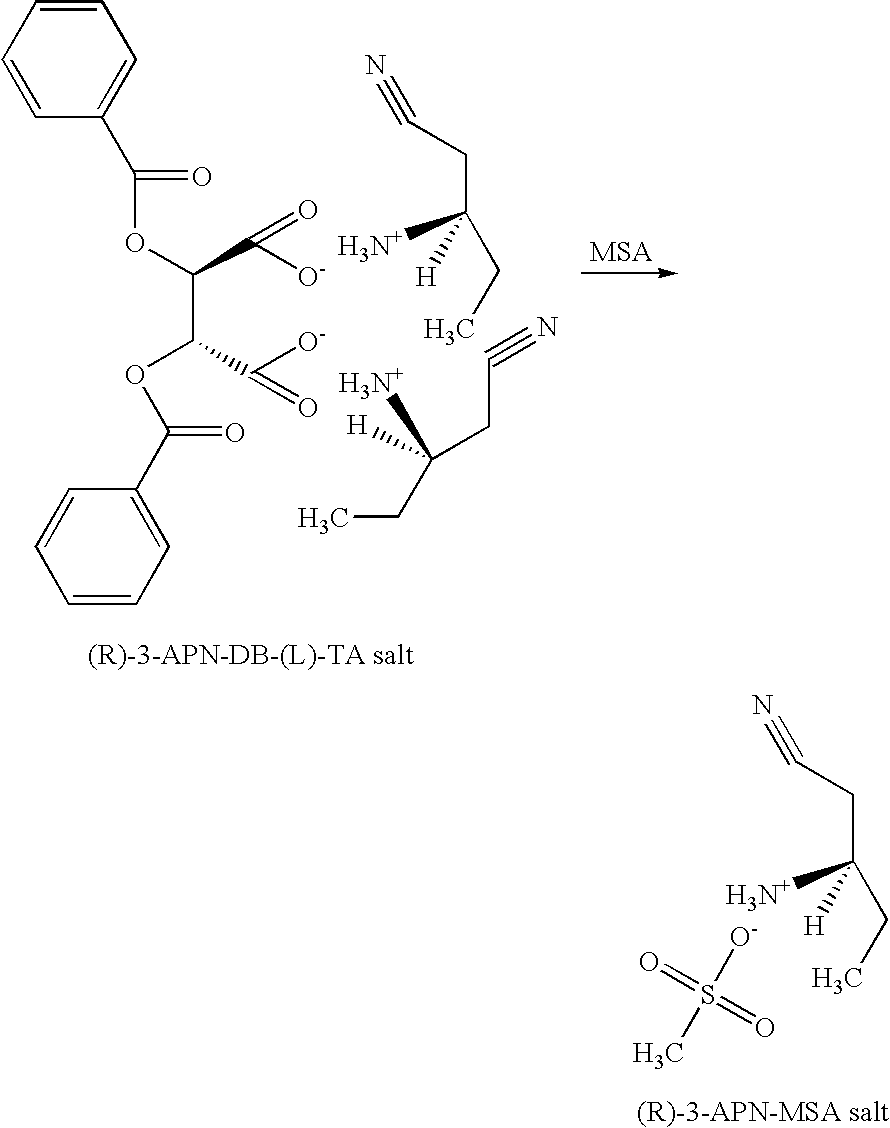
InactiveUS20050038281A1Organic compound preparationOptically-active compound separationNitriteEnantiomer
A process and intermediates for producing 3-amino nitrites. The process involves resolving an enantiomeric mixture of chiral 3-amino nitrites in the presence of a chiral acid in a solvent system to produce a chiral 3-amino nitrile salt. The process may further comprise a recrystalizing step, wherein an enantiomerically enriched 3-amino nitrile salt is produced. The process may further comprise a salt exchanging step, wherein another acid is added to the chiral 3-amino nitrile salt or the enantiomerically enriched 3-amino nitrile salt to produce another 3-amino nitrile salt.
Owner:PFIZER INC
Method for producing aminonitriles
InactiveUS8153845B2Speed up the processIsocyanic acid derivatives preparationCarboxylic acid nitrile preparationAminoacetonitrileCyanohydrin
The invention relates to a process for preparing an amino nitrile mixture comprising aminoacetonitrile (AAN) and from 5 to 70% by weight of iminodiacetonitrile (IDAN), which comprises heating crude AAN which is largely free of formaldehyde cyanohydrin (FACH-free) at a temperature of from 50 to 150° C.
Owner:BASF AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com