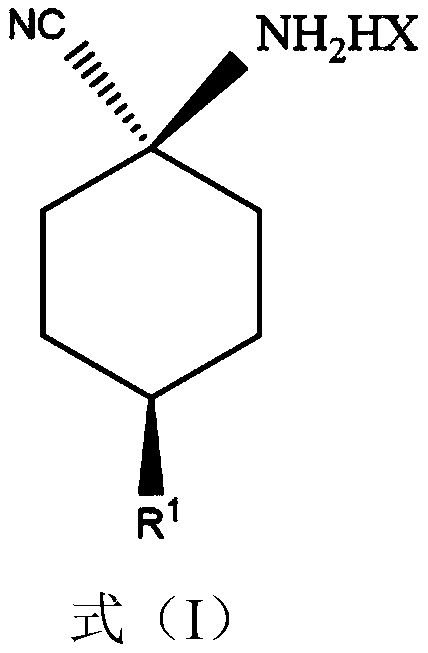
Improved preparing method of cis-counterpoint substituted cyclohexyl amino nitrile maleate
A technology of base amino nitrile and maleate, applied in the field of preparation of cyclohexyl amino nitrile maleate, can solve the problems of polluting products, affecting product quality, low solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] To 602kg cis / trans ratio is the toluene solution (solution total amount is 1500kg) of p-methoxycyclohexylaminonitrile of 55:45 to add 181kg (0.4mol mole times) maleic acid, after adding, maintain temperature at 25~30°C, ultrasonic vibration for 2 hours while stirring, the ultrasonic power is 0.4 W / L, then filter the precipitated maleate, rinse with an appropriate amount of toluene, and dry to obtain white cis-p-methoxycyclohexyl Aminonitrile maleate (386kg, cis / trans ratio: 95.5:4.5).
[0026] Gained product is passed through gas-phase internal standard method, take p-xylene as internal standard substance to measure the content of cyanamide maleate under the following conditions, convert the amount of maleic acid combined with cyanamide by this content, maleic acid Feed amount minus the amount of maleic acid combined with cyanamide is the amount of free maleic acid wrapped.
[0027] Gas phase conditions: FID detector
[0028] Inlet 245°C Detector: 250°C
[0029] Prog...
Embodiment 2
[0035] The time of ultrasonic vibration while stirring was adjusted to 4 hours, and the others were the same as in Example 1 to obtain white cis-p-methoxycyclohexylaminonitrile maleate (404.5kg, cis / trans ratio: 96.8:3.2).
[0036] The same method as in Example 1 records that the free maleic acid that is wrapped accounts for 4.1% of the total amount of maleic acid charged, and the decomposition rate of the obtained cis-p-methoxycyclohexylaminonitrile maleate on the 7th day is 0.37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com