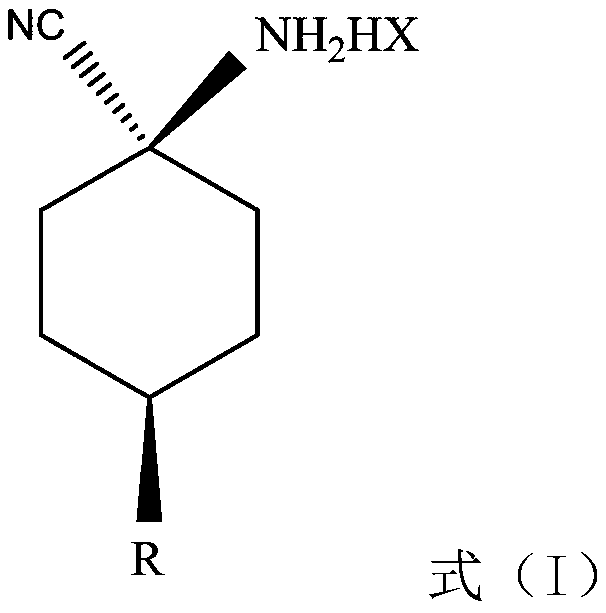
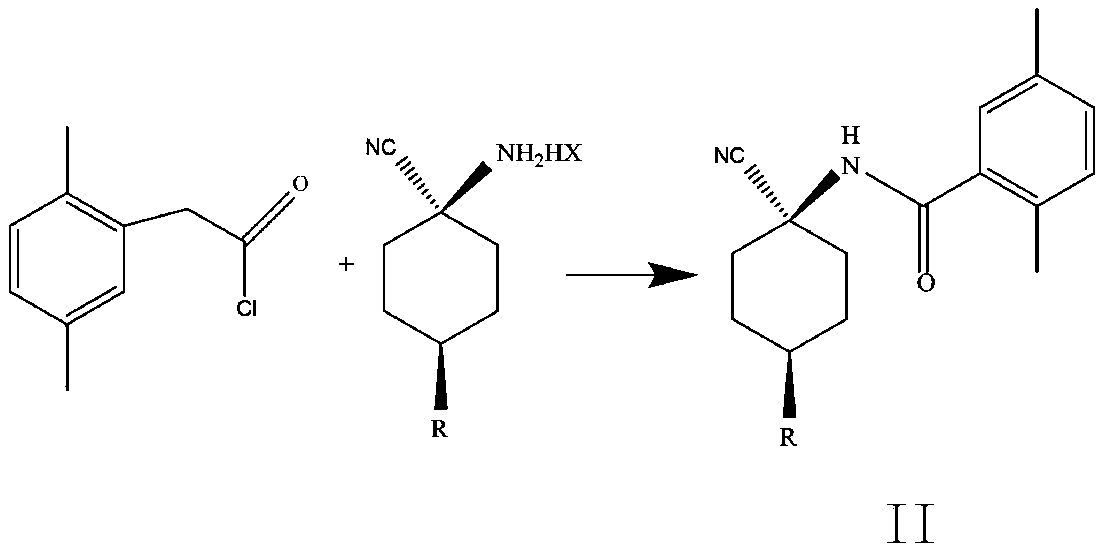
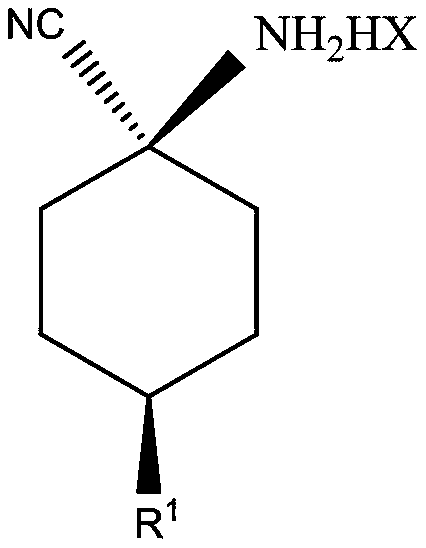
Cis-para-substituted cyclohexylamino nitrile salt and preparation method therefor
A kind of amino nitrile and substituent technology, applied in the field of cis-para-substituted cyclohexyl amino nitrile salt and its preparation, can solve the problem of poor selectivity of toluenesulfonic acid and methanesulfonic acid, affecting the subsequent use of products, and crystallization and separation of cis- Formula isomer compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 cis-p-methoxycyclohexylaminonitrile maleate
[0049]Add 600 grams of ethyl acetate solution of cis / trans-p-methoxycyclohexylaminonitrile (cis:trans=55:45) in the three-necked flask, add 39.2 grams of maleic acid (0.34 mol times), stirred for 8-12 hours, and then cooled to 5-10°C in an ice-water bath to crystallize. The precipitated maleate was filtered and rinsed with an appropriate amount of ethyl acetate. After vacuum drying at 35-40°C, white crystals of cis-p-methoxycyclohexylaminonitrile maleate (75.7 g, cis / trans ratio=95.5:4.5) were obtained.
Embodiment 2
[0050] Embodiment 2 cis-p-methoxycyclohexylaminonitrile maleate
[0051] Add 600 grams of ethyl acetate solution of cis / trans-p-methoxycyclohexylaminonitrile (cis:trans=55:45) in the three-necked flask, add 52.2 grams of maleic acid (0.45 mol times), stirred for 8-12 hours, and then cooled to 5-10°C in an ice-water bath to crystallize. The precipitated maleate was filtered and rinsed with an appropriate amount of ethyl acetate. After vacuum drying at 35-40°C, white crystals of cis-p-methoxycyclohexylaminonitrile maleate (108.1 g, cis / trans ratio=96.1:3.9) were obtained.
Embodiment 3
[0052] Embodiment 3 cis-p-methoxycyclohexylaminonitrile glycolate
[0053] Add 600 grams of ethyl acetate solution of cis / trans-p-methoxycyclohexylaminonitrile (cis:trans=55:45) into the three-necked flask, and add 34.2 hydroxyacetic acid (0.45mol moles) at 25-30°C times), stirred for 8-12 hours, and then cooled to 5-10°C in an ice-water bath to crystallize. The precipitated glycolate was filtered and rinsed with appropriate amount of ethyl acetate. After vacuum drying at 35-40°C, white cis-p-methoxycyclohexylaminonitrile glycolate crystals (92.1 g, cis / trans ratio=94:6) were obtained.
[0054] Comparative Example 1 cis-p-methoxycyclohexylaminonitrile formate
[0055] Add 600 grams of ethyl acetate solution of cis / trans-p-methoxycyclohexylaminonitrile (cis:trans=55:45) to the three-necked flask, and drop 20.7 grams of formic acid (0.45mol Mole times), when the formic acid is added to about half of the amount, a large amount of solids will precipitate out and agglomerate, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com