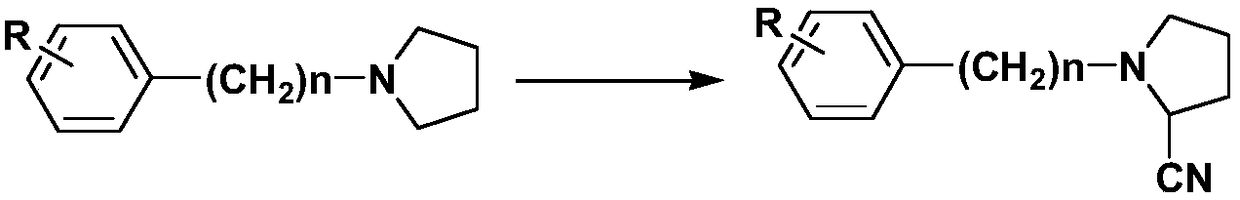
Preparation method of alpha-amino-nitrile compound taking pyrrolidine tertiary amine as primer
A tertiary amine and pyrrolidine technology, which is applied in the field of pharmaceutical intermediates and organic synthesis, can solve the problems of cyanation methods without N-substituted pyrrolidine tertiary amine substrates, and the cyanogen source cannot get rid of toxicity, etc., to achieve High yield, low price, and the effect of overcoming reaction difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] S1: Add 60ml of trifluoroethanol-dichloromethane mixed organic solvent (trifluoroethanol:dichloromethane=36ml:24ml) and tertiary amine raw material N-phenylpyrrolidine 10mmol, Cu(OTf) 2 - Copper acetate composite catalyst 2mg (wherein Cu(OTf) 2 1.2mg), in the presence of 8mmol sodium tert-butoxide, mix and stir at room temperature for 10min, and gradually raise the temperature to 60°C;
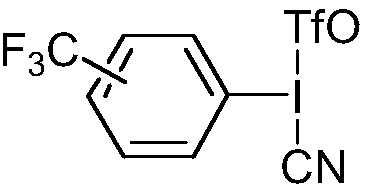
[0055] S2: Add 30mmol cyanide potassium ferrocyanide K 4 Fe(CN) 6 , and 5mmol of the p-trifluoromethyl iodobenzene derivative as a reaction accelerator, nitrogen replacement to remove the air in the reaction system, keep the temperature, fully react under stirring conditions, and use TLC to detect the reaction; wherein, the The structure of trifluoromethyl iodobenzene derivatives is as follows:
[0056]
[0057] S3: after 5h, the reaction is complete, cooled to room temperature, the reaction system is suction filtered to remove the catalyst, the filter cake is washed with dichlor...
Embodiment 2
[0061] S1: Add 100ml trifluoroethanol-dichloromethane mixed organic solvent (trifluoroethanol:dichloromethane=70ml:30ml) and N-phenylpyrrolidine 20mmol, Sc(OTf) respectively in the round bottom flask equipped with stirring 3 -Copper acetate composite catalyst 3mg (wherein Sc(OTf) 3 2mg), in the presence of 16mmol potassium tert-butoxide, mixed and stirred at room temperature for 5min, and gradually raised the temperature to 70°C;
[0062] S2: Add 60mmol cyanide potassium ferrocyanide K 4 Fe(CN) 6 , and 15mmol of 2-iodo-5-cyanotrifluorotoluene as a reaction accelerator, nitrogen replacement to remove the air in the reaction system, keep the temperature, fully react under stirring conditions, and proceed with the TLC detection reaction;
[0063] S3: After the reaction is completed, cool to room temperature, remove the catalyst by suction filtration of the reaction system, wash the filter cake with dichloromethane, collect the combined filtrate, remove the solvent under reduce...
Embodiment 3
[0065] S1: Add 100ml trifluoroethanol-dichloromethane mixed organic solvent (trifluoroethanol:dichloromethane=70ml:30ml) and N-benzylpyrrolidine 20mmol, Cu(OTf ) 2 - 4 mg of indium triiodide composite catalyst (wherein Cu(OTf) 2 3mg), in the presence of 20mmol potassium tert-butoxide, mixed and stirred at room temperature for 5min, and gradually raised the temperature to 60°C;
[0066] S2: Add 60mmol cyanide potassium ferrocyanide, and 20mmol of 2-iodo-5-cyanotrifluorotoluene as a reaction accelerator, replace with nitrogen to remove the air in the reaction system, keep the temperature, under stirring After fully reacting for about 6 hours, the reaction is basically over;
[0067] S3: After the reaction is completed, cool to room temperature, remove the catalyst by suction filtration of the reaction system, wash the filter cake, collect the combined filtrate, remove the solvent under reduced pressure, and separate using silica gel column chromatography (eluent is V (petrole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com