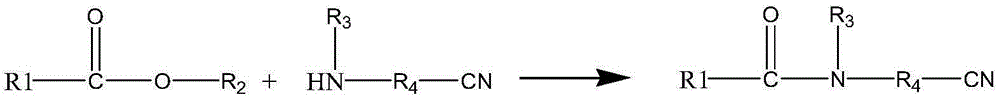
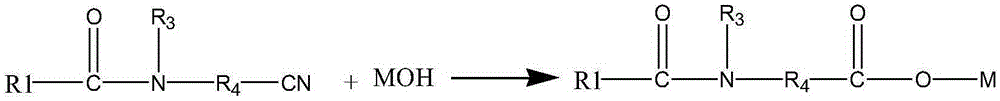
Method for synthesizing N-fatty acyl group amino acid
A fatty acyl amino acid, fatty acid ester technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitriles, etc., can solve the problems of product purity that is difficult to meet industrial needs, complicated operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Example group 1
[0020] This embodiment is implemented in the following ways:
[0021] Step 1) Add 429g (2mol) methyl laurate into a four-necked flask, start stirring, and further add 140g (2mol) aminopropionitrile and 28% sodium methoxide / methanol solution.
[0022] Step 2) Using nitrogen to replace air, the reaction temperature is very slow below 70°C, and the color of the material is darker than 120°C, so the reaction temperature is controlled between 70 and 120°C.
[0023] The effects of catalyst dosage and reaction temperature on the experimental results were obtained through experimental comparison, as shown in Table 1. The optimum reaction temperature was 90°C, and the catalyst addition amount was 5.0% (molar ratio).
[0024] Table 1
[0025]
[0026] Example group 2
[0027] In the present embodiment, concentrated sulfuric acid is added to the reaction solution of the above-mentioned embodiment 3, and the reaction solution is cooled to 0° C. after being ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com