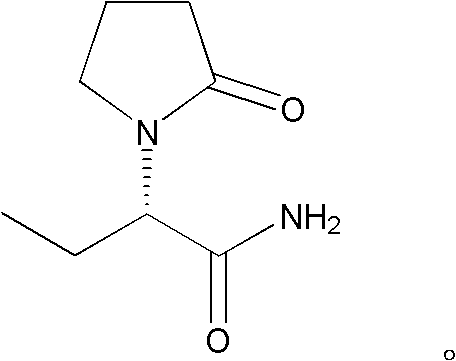
A method for preparing (s)-2-aminobutanamide by enzymatic method
A technology for the preparation of aminobutyramide and enzymatic method, which is applied in the field of preparation of the key intermediate - 2-aminobutanamide, can solve the problems of low yield, large environmental hazards, and high cost of the preparation method, and achieve low process cost and increase production. efficiency and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0013] The synthesis of embodiment 12-aminobutyronitrile 2
[0014] In a 100 ml three-necked flask, add 8.45 grams of potassium cyanide (1.3 equivalents), 40 milliliters of 25% ammonia water, and then add 7.87 grams of ammonium chloride (1.5 moles), and dissolve under stirring. 5.8 g (0.1 mole) of n-propanal (compound 1) was added dropwise, the temperature was controlled at 10-25° C., stirred overnight, and extracted three times with dichloromethane. The organic extracts were combined, washed with 20 ml of saturated brine, and dried by adding 10 g of anhydrous magnesium sulfate. After filtration, dichloromethane was removed by rotary evaporation in a water bath at 30°C under reduced pressure to obtain about 6.7 g of 2-aminobutyronitrile as light yellow oily liquid with a yield of 79.8%.
Embodiment 2
[0015] The screening of embodiment 2 nitrile hydratases
[0016] The 2-aminobutyronitrile obtained in Example 1 is formulated into a solution of 10 grams per liter, and 24 kinds of nitrile hydratases of 5 mg NHT-101~NHT-124 are added respectively in parallel reaction vessels, and 0.45 milliliters of phosphate buffer saline is added (Na 2 HPO 4 / NaH 2 PO 4 , 0.1M, pH 7.2), and then add 0.05 ml of α-aminobutyronitrile solution, shake overnight at room temperature, and detect the reaction by LC-MS. As a result, 13 enzymes can react to produce products. These 13 enzymes are carried out selective comparison, control reaction time, make its reaction conversion rate 80% (CR (+) column; UV 200nm detection; mobile phase: perchloric acid aqueous solution (pH 1.2)), the three enzymes were used to prepare (S)-2-aminobutyramide.
Embodiment 3
[0017] The preparation of embodiment 3 (S)-2-aminobutanamide (ES-NHT-105)
[0018] Add 50 mg of 2-aminobutyronitrile, 1 mg of nitrile hydratase NHT-105, and 5 ml of phosphate buffer (Na 2 HPO 4 / NaH 2 PO 4 , 0.1M, pH 7.2), after reacting on a rotary shaker at 160 rpm at 30°C for 15 hours, samples were taken for HPLC analysis, the conversion rate was 33%, and the ee value was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com