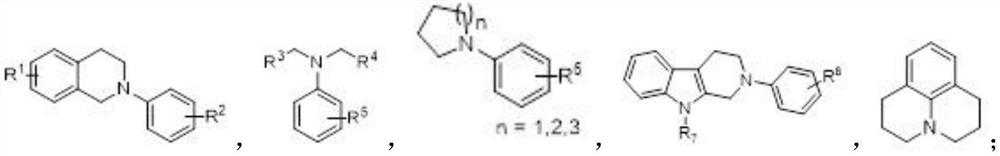
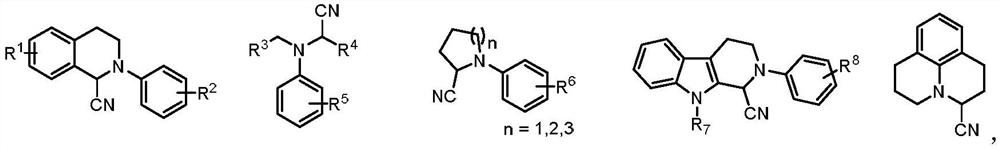
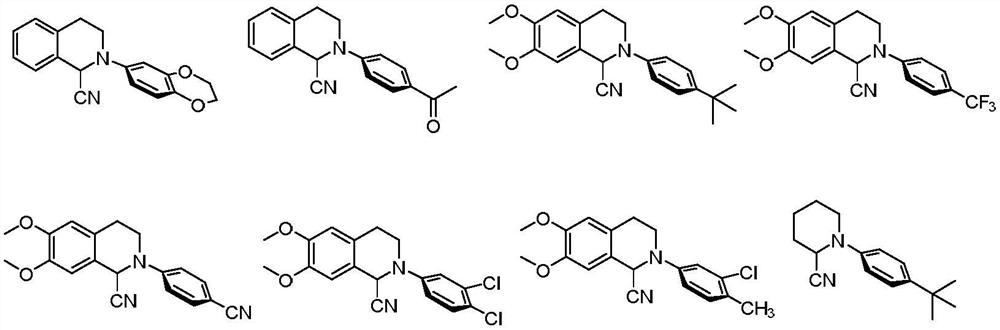
Method for preparing alpha-aminonitrile and product and application thereof
A technology of amino nitrile and amino group, applied in the field of preparing α-amino nitrile, can solve the problems of toxicity and cost that cannot be ignored, increased production cost and high preparation cost, and achieves the effects of low toxicity, short reaction time and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 (do not add alkali)
[0041] Synthetic method: Add 2mmol of tertiary amine 1 into a 100mL reaction flask, then add 2mmol of benzoylnitrile and 40mL of acetonitrile, vacuumize the reaction device and pass in argon, and magnetically Stir the reaction. The reaction vial was placed at a distance of 1 cm from an 18W purple light with a wavelength of 390-400nm, and the reaction was continued for 24 hours. After the reaction was completed, silica gel was used for adsorption and the solvent was spin-dried in vacuum. The eluent was petroleum ether / ethyl acetate system (volume ratio: 20:1). The results are shown in Table 1. The reaction formula is as follows:
[0042]
[0043] Table 1
[0044]
[0045]
Embodiment 2-6
[0046] Embodiment 2-6 (different light sources)
[0047] Synthesis method: 2mmol of tertiary amine 1 is added to a 100mL reaction flask, then 3mmol of benzoyl nitrile, 2mmol of lithium carbonate or other bases such as those in Table 2, and 40mL of acetonitrile are added, the reaction device is evacuated and Argon gas was introduced, and the reaction was magnetically stirred at room temperature under the protection of argon gas. Place the reaction bottle at a distance of 1cm from 18W 365-375nm ultraviolet light source, 380-390nm purple light source, 400-415nm purple light source, 450-465nm blue light source or 510-520nm green light source, and continue to react for 24 hours. After the reaction was completed, silica gel was used for adsorption and the solvent was spin-dried in vacuum. The eluent was petroleum ether / ethyl acetate system (volume ratio: 20:1). The results are shown in Table 2. The reaction formula is as follows:
[0048]
[0049] Table 2
[0050]
Embodiment 7-27
[0052]Synthesis method: Add 2mmol of tertiary amine 1 into a 100mL reaction flask, then add 3mmol of benzoyl nitrile, 2mmol of lithium carbonate, and 40mL of acetonitrile, vacuumize the reaction device and pass it into argon, Under protective conditions, the reaction was stirred magnetically at room temperature. The reaction vial was placed at a distance of 1 cm from an 18W purple light with a wavelength of 390-400nm, and the reaction was continued for 24 hours. After the reaction was completed, silica gel was used for adsorption and the solvent was spin-dried in vacuum. The eluent was petroleum ether / ethyl acetate system (volume ratio 10-40:1). The results are shown in Table 3. The reaction formula is as follows:
[0053]
[0054] table 3
[0055]
[0056]
[0057]
[0058]
[0059]
[0060]
[0061] In the above examples, lithium carbonate is replaced by 2,6-lutidine, pyridine, 1,8-diazabicycloundec-7-ene, N-methylmorpholine, Hans ester, phosphoric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com