Preparation method of 3-aminomethyl-3,5,5-trimethylcyclohexylamine
一种三甲基环己胺、三甲基环己酮的技术,应用在脂肪族胺的制备领域,能够解决催化剂成本提高、反应器体积增大、氨基腈低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
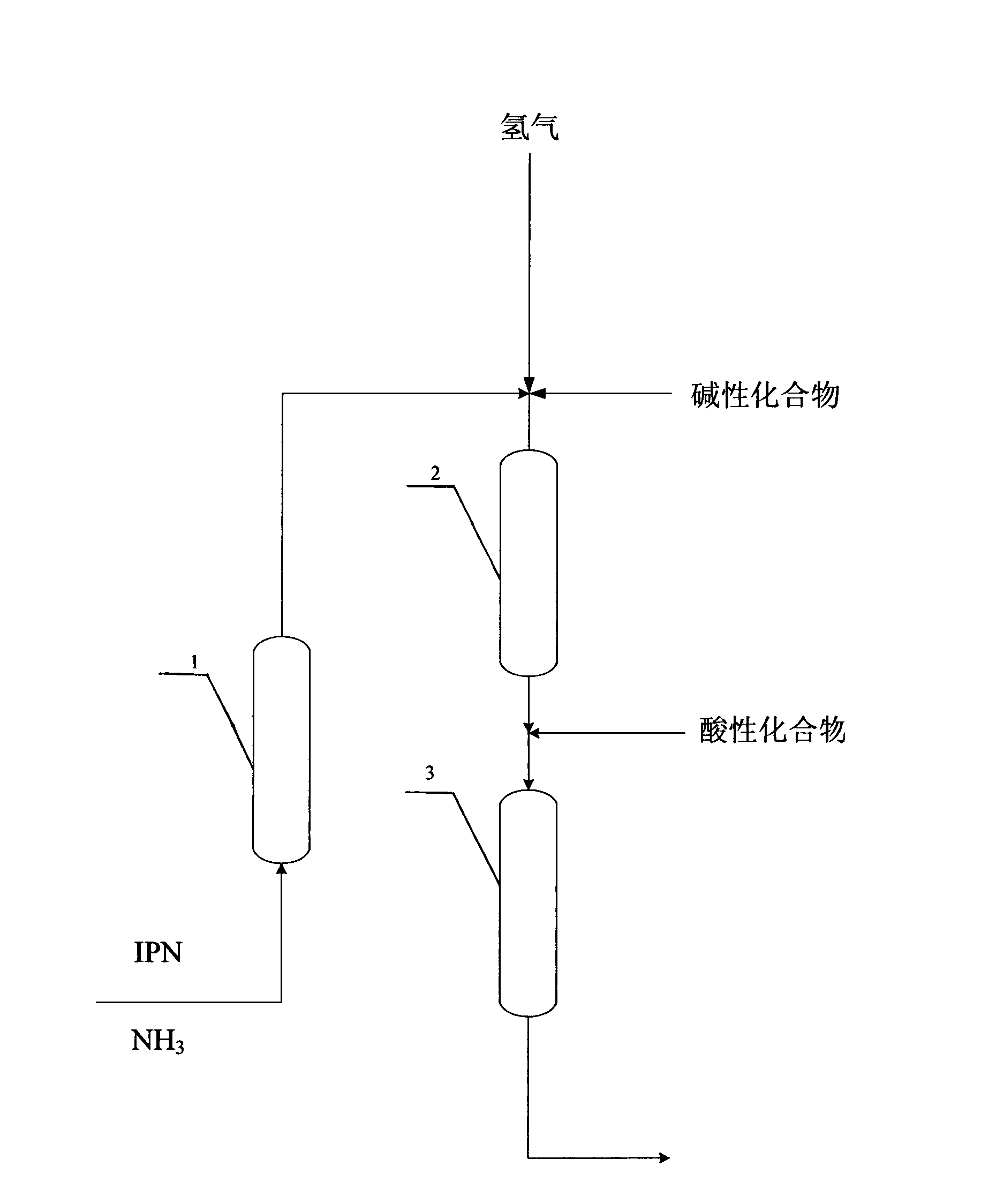
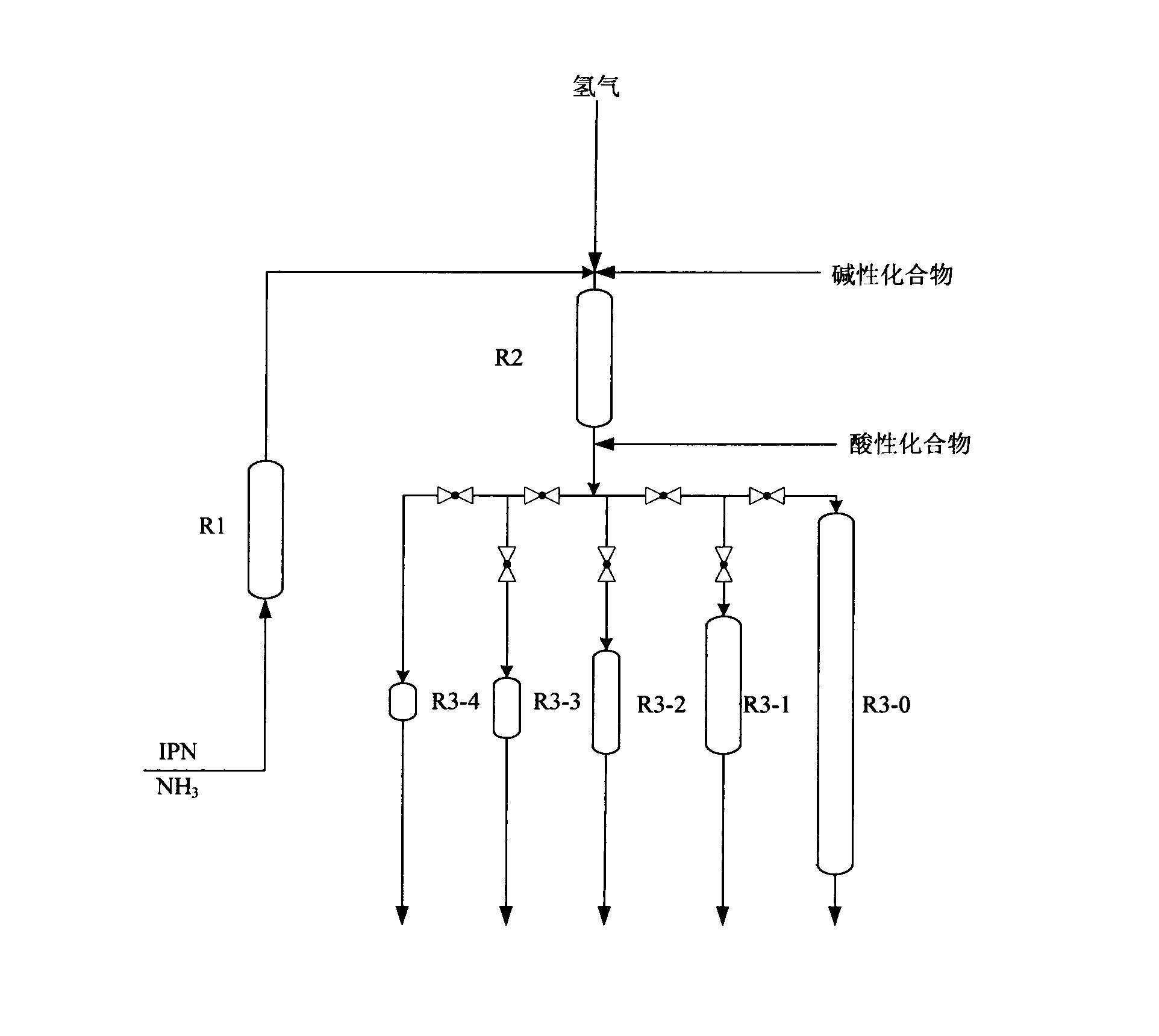
[0054] process such as figure 2 As shown, the temperature of R1 is controlled at 40°C, the temperature of R2 is controlled at 60°C, the temperature of R3-0 is controlled at 130°C, the reaction pressure of R1, R2, and R3-0 is all controlled at 15MPa, the feed rate of IPN is 80g / h, and the NH 3 The feed rate is 168g / h, and the hydrogen flow rate is 1100 standard L / h. Before the material flow enters into R2, a NaOH methanol solution with a mass concentration of 5% is added, and the feed rate is 16 g / h. The space velocity over the catalyst in each reactor is as follows:
[0055]
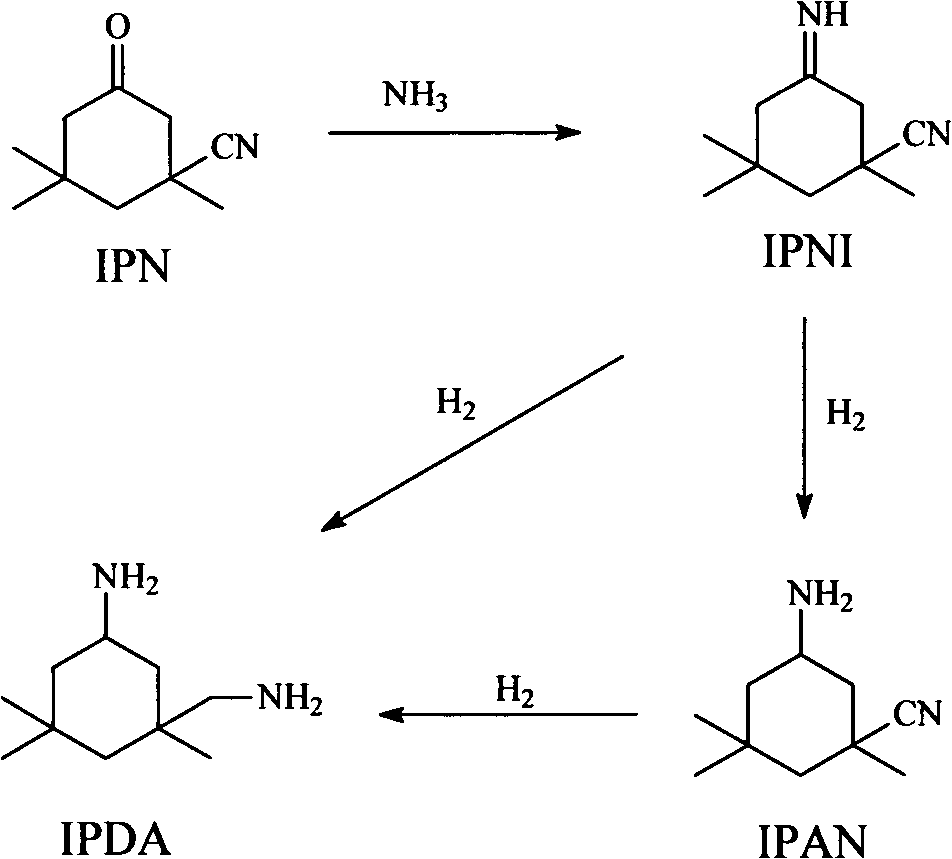
[0056] After the device was running for 100 hours, before the reaction mixture entered R3-0, a methanol solution of formic acid with a mass concentration of 5% was added to the reaction mixture, and the feed rate was 16 g / h. After device operation 200 hours, take a sample again from R1 reactor outlet, wherein the content of 3-cyano-3,5,5-trimethylcyclohexylimine is 95%, take a sample again from R2 ...
Embodiment 2
[0059] process such as figure 2 As shown, the temperature of R1 is controlled at 40°C, the temperature of R2 is controlled at 60°C, the temperature of R3-0, R3-1, R3-2, R3-3, and R3-4 is controlled at 130°C, and the temperature of R1, R2, R3-0, The reaction pressures of R3-1, R3-2, R3-3, and R3-4 are all controlled at 15MPa, the feed rate of IPN is 80g / h, and the NH 3 The feed rate is 168g / h, and the hydrogen flow rate is 1100 standard L / h. Before the material flow enters into R2, a NaOH methanol solution with a mass concentration of 5% is added, and the feed rate is 16 g / h. Before the reaction mixture enters the second-stage hydrogenation reactor, a methanol solution of formic acid with a mass concentration of 5% is added to the reaction mixture, and the feed rate is 16 g / h. The space velocity over the catalyst in each reactor is as follows:
[0060]
[0061]
[0062] When the test device reaches 300 hours, 400 hours, 500 hours, and 600 hours, the products that have u...
Embodiment 3
[0067] The temperature of R1 is controlled at 60°C, the temperature of R2 is controlled at 100°C, the temperature of R3-0 is controlled at 100°C, the reaction pressure of R1, R2, and R3-0 is all controlled at 20MPa, the feed rate of IPN is 80g / h, and the NH 3 The feed rate is 650g / h, and the hydrogen flow rate is 220 standard L / h. Before R2, a LiOH dimethyl ether solution with a mass concentration of 1% was added, and the feed rate was 8 g / h. Before the reaction mixture enters the R3-0, an aqueous solution of phthalic acid with a mass concentration of 1% is added to the reaction mixture, and the feed amount is 8 g / h.
[0068] After device operation 100 hours, take a sample from R1 reactor outlet, do gas chromatographic analysis, wherein the content of 3-cyano-3,5,5-trimethylcyclohexyl imine is 94%, R2 reactor outlet sampling, Do gas chromatography analysis, wherein the IPDA content is 81%, and the aminonitrile content is 14.5%.
[0069] At the beginning of the test, use R3-0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com