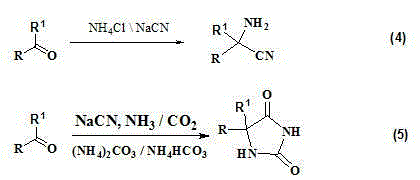
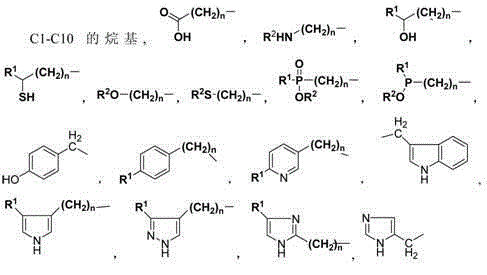
Clean synthesis process of alpha-amino acid compounds
A technology of amino acid and synthesis process, which is applied in the preparation of organic compounds, compounds of group 5/15 elements of the periodic table, cyanide reaction preparation, etc. It can solve problems such as difficult separation and achieve good purity and high yield , the effect of very little amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 2
[0046] Example 2: Synthesis of 2-amino-3-(4-imidazolyl)propionic acid
[0047] Add 90g (0.5mol) of 5-[(4-imidazolyl)methyl]hydantoin into a 1L autoclave, add 240g (2.5mol) of ammonium carbonate, 300g of water, and heat to 150-170 o C, pressure 5~8MPa, react for 5h, distill out ammonium carbonate at normal pressure, then distill out water under reduced pressure, add 150g methanol to the residue and reflux for 2h, cool down, and crystals are precipitated. Filtration, vacuum 120 o C dried for 4 hours to obtain 62.0 g of 2-amino-3-(4-imidazolyl)propionic acid with a purity of 96% and a yield of 80.0%.
Embodiment example 3
[0048] Example 3: Synthesis of 2-amino-3-(4-imidazolyl)propionic acid
[0049] Add 90g (0.5mol) of 5-[(4-imidazolyl)methyl]hydantoin to a 1L autoclave, add 197.5g (3mol) of ammonium bicarbonate, 170g (2mol) of 20% ammonia solution, triethylbenzyl chloride Ammonium chloride 3g, water 200g, heated to 150-170 oC, pressure 5~7MPa, react for 4 hours, distill ammonium bicarbonate and ammonia water under normal pressure, add 150g methanol to the residue and reflux for 2 hours, cool down, and crystals are precipitated. Filtration, vacuum 120 o C dried for 4 hours to obtain 62.7 g of 2-amino-3-(4-imidazolyl)propionic acid with a purity of 97% and a yield of 80.9%.
Embodiment example 4
[0050] Example 4: Synthesis of 2-amino-3-(4-imidazolyl)propionic acid
[0051] Add 90g (0.5mol) of 5-[(4-imidazolyl)methyl]hydantoin into a 1L autoclave, add 255g of 20% ammonia water (3mol), 3g of triethylbenzyl ammonium chloride, and 300g of water, and heat to 150- 170 o C, pressure 5~8MPa, react for 4h, drop to room temperature, concentrate the mother liquor under reduced pressure, add the residue to 150g ethanol and reflux for 2h, cool down, and crystals precipitate out. Filtration, vacuum 120 o C dried for 4 hours to obtain 53.5 g of 2-amino-3-(4-imidazolyl)propionic acid with a purity of 96% and a yield of 69.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com