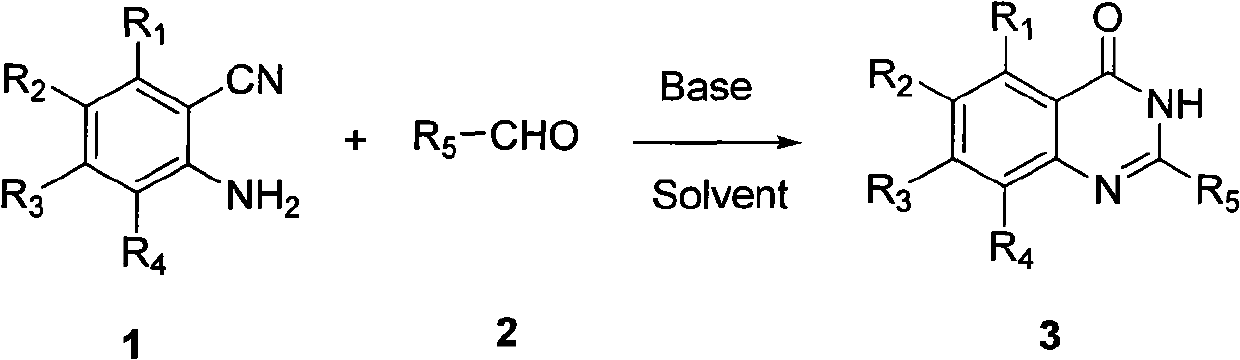
Method for synthesizing quinazoline-4-(3H)-ketone
A technology for synthesizing quinazoline and crystallization, which is applied in the field of drug synthesis, can solve the problems of complex post-processing, poor stability, and long reaction time, and achieve the effects of wide application range, mild reaction conditions, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 20mL of dried toluene to a 100ml single-necked bottle, add 10mmol of 2-aminobenzonitrile under stirring, then add 15mmol of p-chlorobenzaldehyde, 2mmol of sodium hydroxide, and heat to reflux for 2h. After the reaction was completed, the reaction solution was poured into 20 mL of aqueous solution, extracted with ethyl acetate, and dried by rotary evaporation. Then crystallize with ethanol to obtain the product 2-(4-chlorophenyl)quinazolin-4(3H)-one. Yield 83%, Mp.>300°C.
[0029]
[0030] 1 H NMR (DMSO-d 6 , 400MHz) δ: 7.54-7.61 (m, 2H, ArH), 7.67 (d, J=7.6Hz, 1H, ArH), 7.77 (d, J=8.0Hz, 1H, ArH), 7.84-7.88 (m, 1H, ArH), 8.14-8.18(m, 2H, ArH), 8.24(s, 1H, ArH), 12.64(s, 1H, NH). MS(ESI): m / z(%)=257.0(100) [M+H] +
Embodiment 2
[0032] Use acetaldehyde instead of p-chlorobenzaldehyde, and the others are the same as in Example 1 to obtain the target product 2-methylquinazolin-4(3H)-one. Yield 75%. Mp.238-240°C.
[0033]
[0034] 1 H NMR (DMSO-d 6 , 300MHz) δ: 12.19 (s, br, 1H), 8.07 (d, 1H, J=8.1Hz), 7.77 (t, 1H, J=7.5Hz), 7.56 (d, 1H, J=7.9Hz), 7.45(t, 1H, J=7.5Hz), 2.36(s, 3H); 13 C NMR (DMSO-d 6 , 75MHz) δ: 162.2, 154.8, 149.5, 134.8, 127.1, 126.4, 126.2, 121.2, 21.9. MS (ESI) m / z (%) = 161.2 (100) [M+H] +
Embodiment 3
[0036] Replace 2-aminobenzonitrile with 5-nitro-2-aminobenzonitrile, replace p-chlorobenzaldehyde with p-methoxybenzaldehyde, and others are the same as in Example 1 to obtain target product 2-(4-methoxybenzene base)-6-nitroquinazolin-4(3H)-one, the yield was 71%. Mp.>300°C.
[0037]
[0038] 1 H NMR (DMSO-d 6 )δ: 12.83(s, 1H, NH), 8.80(d, 1H, J=2.4Hz, ArH), 8.53(dd, 1H, J=2.4, 8.0Hz, ArH), 8.25(d, 2H, J= 8.0Hz, ArH), 7.86(d, 1H, J=8.0Hz, ArH), 7.12(d, 2H, J=8.0Hz, ArH), 3.85(s, 1H, CH 3 ); 13 C NMR (DMSO-d 6)δ: 55.6, 114.2, 114.2, 114.2, 120.6, 122.1, 124.0, 128.5, 130.2, 130.2, 130.2, 144.3, 155.2, 161.7, 162.7; MS (ESI): m / z (%) = 298.1 (100) [ M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com