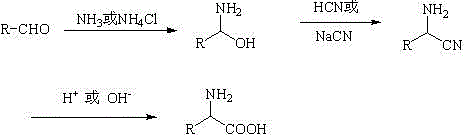
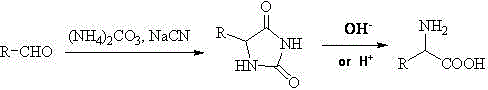
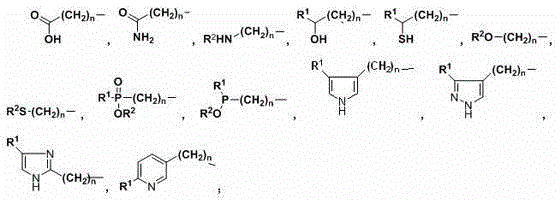
Synthesis and purification method for alpha-amino acid compound
An amino acid and purification method technology, which is applied in the field of synthesis and purification of α-amino acid compounds, can solve problems such as difficulty in separation, and achieve the effects of high purity, recycling and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0061] Example 1: Synthesis of 2-amino-4-methylthiobutyric acid
[0062] Add 130g (1mol) of 2-amino-4-methylthiobutyronitrile into a 1L autoclave, add 68g of 25% ammonia water and 56g (1mol) of calcium oxide, 2g of tetrabutylammonium bisulfate, 500g of water, and heat to 180-190 o C, pressure 2.5-3.0MPa, react for 5h, cool down to room temperature, pass carbon dioxide under stirring until pH = 8, filter, concentrate the mother liquor under reduced pressure, add 150g methanol to the residue and reflux for 2h, cool down, and crystals precipitate out. Filtration, vacuum 120 o C dried for 4 hours to obtain 118.3 g of 2-amino-4-methylthiobutyric acid with a purity of 96% and a yield of 79.4%.
Embodiment example 2
[0063] Example 2: Synthesis of 2-amino-3-(4-imidazolyl)propionic acid
[0064] Add 180g (1mol) of 5-[(4-imidazolyl)methyl]hydantoin to a 1L autoclave, add 68g of 25% ammonia water and 110g (1.5mol) of calcium hydroxide, 500g of water, and heat to 160-170 o C, pressure 1.5~2.0MPa, react for 8h, cool down to room temperature, pass carbon dioxide under stirring until pH=8, filter, concentrate the mother liquor under reduced pressure, add 150g methanol to the residue and reflux for 2h, cool down, and crystals precipitate out. Filtration, vacuum 120 o C dried for 4 hours to obtain 124.2 g of 2-amino-3-(4-imidazolyl)propionic acid with a purity of 96% and a yield of 80.1%.
Embodiment example 3
[0065] Example 3: Synthesis of 2-amino-3-(4-imidazolyl)propionic acid
[0066] Add 180g (1mol) of 5-[(4-imidazolyl)methyl]hydantoin to a 1L autoclave, add 68g of 25% ammonia water and 110g (1.5mol) of calcium hydroxide, 3g of triethylbenzyl ammonium sulfate, and 500g of water , heated to 160-170 o C, pressure 1.5~2.0MPa, react for 3h, cool down to room temperature, pass carbon dioxide to pH=8, filter, concentrate the mother liquor under reduced pressure, add 150g methanol to the residue and reflux for 2h, cool down, and crystals precipitate out. Filtration, vacuum 120 o C dried for 4 hours to obtain 134.5 g of 2-amino-3-(4-imidazolyl)propionic acid with a purity of 96% and a yield of 86.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com