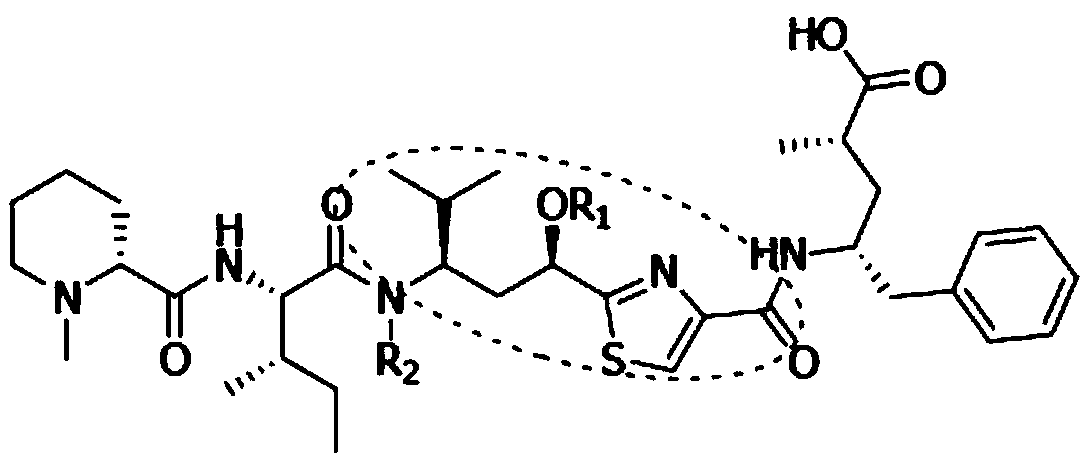
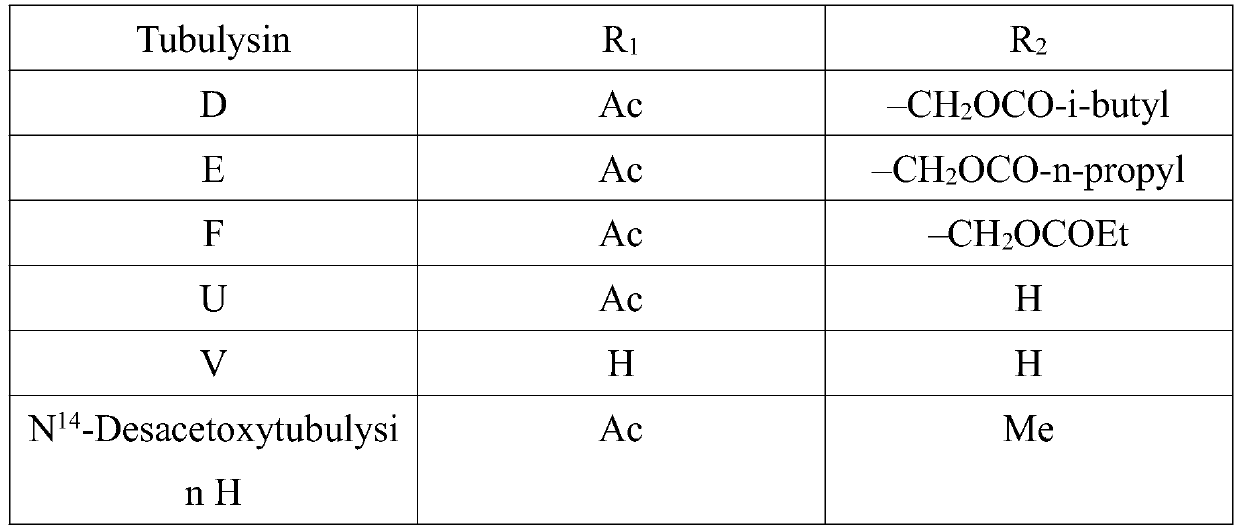
Synthetic method of key intermediate Tuv of natural anti-cancer drug Tubulysins
A synthesis method and technology of anticancer drugs, applied in organic chemistry and other fields, can solve the problems of low practicability, difficult scale-up synthesis, cumbersome operation, etc., and achieve the effect of low cost, simple post-treatment process, and good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
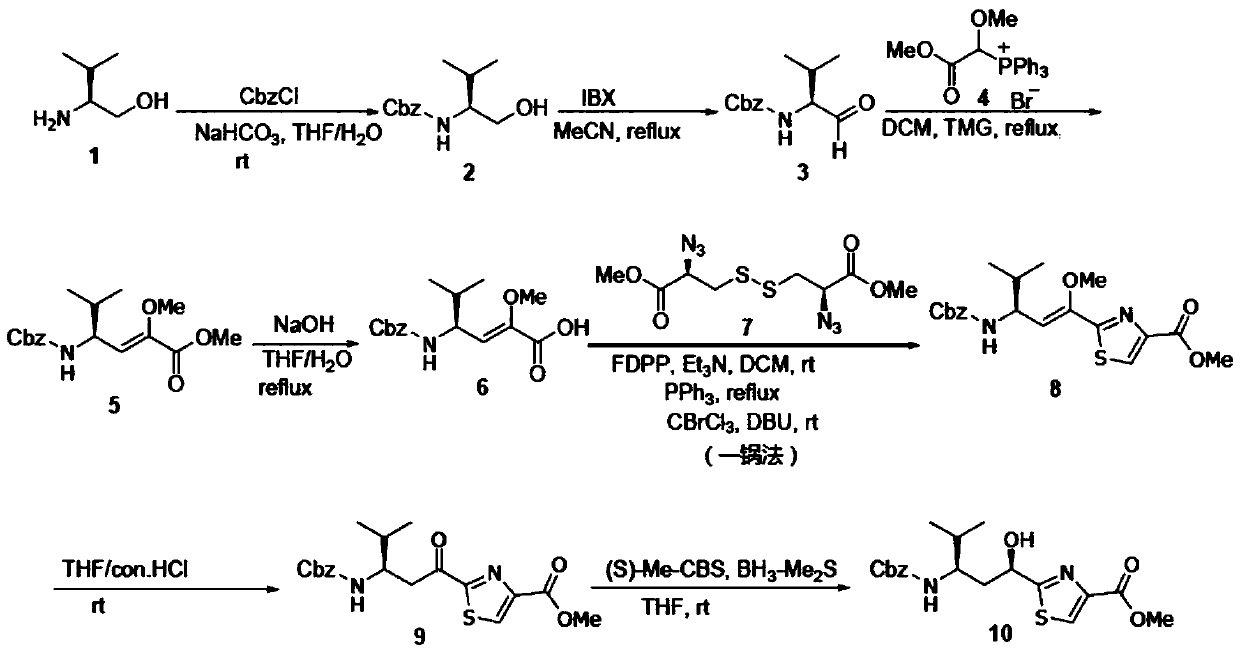
[0063] The present invention provides a method for synthesizing Tuv, a key intermediate of natural anticancer drug Tubulysins, and the route of the synthesis method is as follows:
[0064]
[0065] Including the following steps:
[0066] Step 1. Dissolve the starting material L-valinol 1 in a tetrahydrofuran / water mixed solvent, add solid sodium bicarbonate and benzyl chloroformate CbzCl, and react at room temperature overnight to obtain compound 2;
[0067] Step 2. Dissolve compound 2 obtained in step 1 in acetonitrile, add 2-iodoyl benzoic acid, and react with heating under reflux to obtain intermediate aldehyde 3;
[0068] Dissolve the intermediate aldehyde 3 in dichloromethane, add Wittig reagent 4 and tetramethylguanidine, and react under reflux with heating to obtain compound 5;
[0069] Step 3. Dissolving the compound 5 in a mixed solvent of tetrahydrofuran / water, adding solid sodium hydroxide, and heating and refluxing to react to obtain compound 6;
[0070] Using compound 6 as t...
Embodiment approach
[0080] As an embodiment, in the step 3, the molar ratio of compound 5: sodium hydroxide: compound 7: FDPP: triethylamine: triphenylphosphine: DBU: chlorobromomethane is 1:10-20:0.5- 0.55:1-1.2:2-3:5-6:3-4:2-3, preferably 1:15:0.5:1:2:5:3:2.
[0081] As an embodiment, the compound 5 is reacted with sodium hydroxide for 4-6 hours, preferably for 4 hours. After the reaction, it is concentrated under reduced pressure, acidified with dilute hydrochloric acid, extracted with ethyl acetate three times, and the combined organic phase is washed with saturated brine , Liquid separation, the organic phase was dried with anhydrous sodium sulfate, filtered and concentrated to obtain compound 6;
[0082] Then dissolve compound 6 in dichloromethane, add FDPP (that is, pentafluorophenyl diphenyl phosphate) and triethylamine, react at room temperature for 0.5 to 1 h, preferably 0.5 h, then add compound 7 and triphenyl Phosphine, heat reflux for reaction for 7-12h, preferably 10h, then cool to room...
Embodiment 1
[0088] Example 1: Synthesis of Compound 2
[0089]
[0090] Dissolve L-valinol 1 (20g, 193.9mmol) in a mixed solvent of tetrahydrofuran / water (1:1, 800mL), add sodium bicarbonate (50.4g, 600mmol), stir well, and cool to 0°C in an ice-water bath Benzyl chloroformate, CbzCl (27.3mL, 193.9mmol) was slowly added dropwise. After 30 minutes, the temperature was raised to room temperature and the reaction was stirred for 12h, concentrated under reduced pressure, diluted with water (200mL), and extracted three times with ethyl acetate (300mL). Combine the organic phases. , Dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain compound 2, 43.7 g of white solid, with a yield of 95%. Used directly in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com