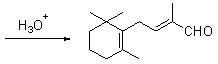
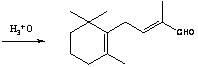
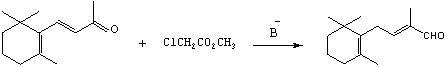Preparation method of C-14 enol ether
A C-14, enol ether technology, applied in ether preparation, organic chemistry and other directions, can solve the problems of difficult synthesis, pollution, difficulty in the source of raw material C-4 phosphonates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of diethyl methoxymethylphosphonate (8).
[0046]In a 1000mL four-neck flask equipped with mechanical stirring, add 455g of triethyl phosphonite, start stirring and heat to 60°C-70°C, and add 80.5g of chloromethyl methyl ether dropwise within 1h. GC tracking, the reaction was completed in about 2 hours. Cool down, recover the solvent, distill the residue under reduced pressure, collect 165g of 102-103°C / 9mmHg fraction, it is a colorless transparent liquid, the gas phase content is 98.5%, and the yield is 89.2%.
[0047] .
[0048] 1 HNMR (δ, ppm, 400MHz , CDCl 3 ): 1.36(t, 6H, CH 2 CH 3 ); 3.48(s, 3H, CH3); 3.74(d, 2H, -CH2-); 4.15-4.22(m, 4H, CH 2 CH 3 ).
[0049] 13 CNMR (400MHz, CDCl 3 )δ (ppm): 16.45, 16.51, 61.30, 61.43, 62.44, 65.93, 67.58.
Embodiment 2
[0050] Example 2:: Preparation of C-14 Enol Ether (7).
[0051] In a nitrogen-protected 250mL three-neck flask, add 12.3g of potassium tert-butoxide (0.11mol) and 50mL of a 8:1 (v / v) mixture of tetrahydrofuran and dimethyl sulfoxide, keep warm in a cold bath, and under mechanical stirring, drop Add 18.2g diethyl methoxymethylphosphonate (8) (0.1 mole), keep at -30~-25°C for about half an hour to complete the dropwise addition, continue to keep warm and stir for about 1 hour to make the carbanion dissociation reaction fully, and then keep Add 19.2g of β-ionone (4) (0.1mol) dropwise at -30~-25℃, and the dropwise addition is completed in about 1 hour. Continue to keep warm and stir for about half an hour. After the reaction is completed by gas chromatography, add 50mL of water and 100mL of ether and stir for 10min. Layered, the ether layer was washed 3 times with 5% aqueous sodium chloride solution (25 mL each time), the organic layer was dried over magnesium sulfate and filtered...
Embodiment 3-9
[0058] Example 3-9: Preparation of C-14 Enol Ether (7) by Condensation Reaction under Different Bases, Solvents and Temperature Conditions.
[0059] In a 250mL three-neck bottle protected by nitrogen, add a certain amount of alkali and 20mL of a certain solvent (see the table below for the types of alkali and solvent), keep warm in a cold bath, and add a certain amount of diethyl methoxymethylphosphonate dropwise under mechanical stirring. (8) 20mL of a certain solvent (see the table below for the molar quantity) (same as the above solvent), keep a certain temperature for about half an hour to complete the dropwise addition, continue to keep warm and stir for about 1 hour to make the carbanion dissociation reaction fully, and then keep the above temperature Add 9.6g of β-ionone (4) (0.05mol) into the solvent (same as the above solvent) dropwise, and the dropwise addition is completed in about 1 hour, continue to keep warm and stir for about half an hour, after the reaction is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com