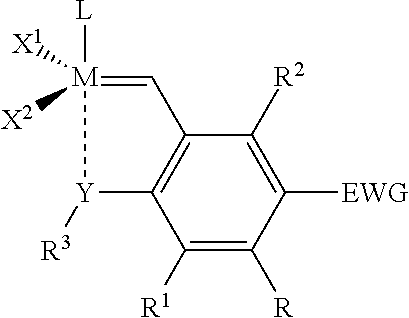
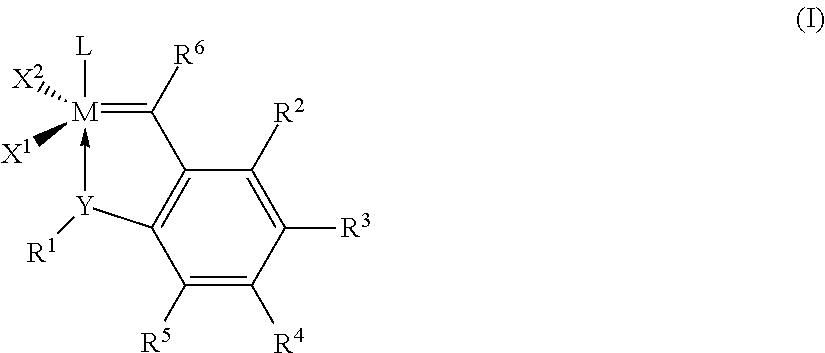
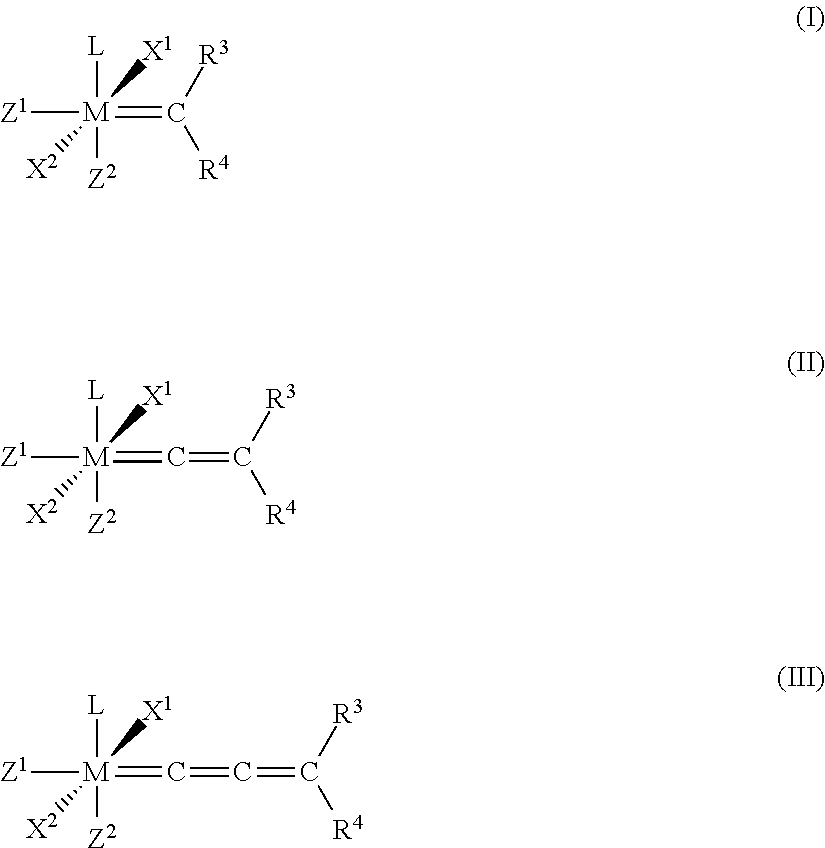Catalyst compositions and their use for hydrogenation of nitrile rubber
a technology of nitrile rubber and catalyst composition, which is applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical apparatus and processes, etc., can solve the problem of certain degree of cross-linking, high price of catalyst metal, and high cost of catalyst metal removal/recycle. achieve high hydrogenation activity, high hydrogenation degree, and constant molecular weight of nitrile rubber during hydrogenation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison Example, Using Catalyst (4)
[0421]A solution of 18 g Perbunan® 3431VP in 282 g MCB (Perbunan® 3431VP concentration of 6 wt %) was bubbled with nitrogen in a 600 mL Parr autoclave for 30 minutes, and then heated to 120° C. Wilkinson's catalyst (15 mg) and PPh3 (18 mg) was dissolved in another 22 g of degassed MCB and then added into the reactor. Hydrogenation was conducted under 4.137 MPa of hydrogen pressure and 800 rpm of agitation speed. Samples were taken from the reactor at intervals for FT-IR analysis to determine the hydrogenation degree. After 5 hours of hydrogenation, the hydrogenation degree reached 90.3%, the reactor was cooled to room temperature and the pressure was released. The final molecular weights and PDI were: Mn=76,286, Mw=260,572, PDI=3.42.
examples 2
Inventive Example; Perbunan 3431VP; Catalyst (1); VEE as Co-Catalyst
[0422]Catalyst (1) (9 mg) was dissolved in 22 g degassed MCB in a flask. Vinyl ethyl ether (100 μL) was injected into the flask and the solution was stirred for 12 hours. A solution of 18 g Perbunan® 3431VP in 282 g MCB (Perbunan®3431VP concentration of 6 wt %) was bubbled with nitrogen in a 600 mL. Parr autoclave for 30 minutes, and then heated to 120° C. The catalyst solution in the flask was transferred into the reactor via syringe. Hydrogenation was conducted under 4.137 Mpa of hydrogen pressure and 800 rpm of agitation speed. Samples were taken from the reactor at intervals for FT-IR analysis to determine the hydrogenation degree. After 3 hours of hydrogenation, the hydrogenation degree reached 93%. The final molecular weights and the PDI were:: Mn=75,844, Mw=223,863, PDI=2.95.
example 3
Inventive Example; Perbunan 3431VP; Catalyst (2); VEE as Co-Catalyst
[0423]All the conditions and operation were the same as in Example 5 except that Catalyst (2) was used (18 mg). The hydrogenation degree at 1 hour was 99%. The final molecular weights and the PDI were: Mn=71,762, Mw=221,604, PDI=3.09.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com